 In February of 2020, Turner S. Baker, Ph.D. Candidate and Project Manager at Sinai BioDesign and Dr. Christopher Kellner, Assistant Professor and Director of the Intracerebral Hemorrhage Program were chosen from a highly competitive pool of applicants to participate in the JHU-Tufts 2020 Design Lab. The Design Lab, jointly run by the Tufts Institute for Clinical Research and Health Policy Studies, the MIT Center for Biomedical Innovation, and the CTSA Trial Innovation Network, explores innovations in clinical trial design in the context of the full treatment development pathway, with a particular focus on innovative clinical effectiveness trial design.
In February of 2020, Turner S. Baker, Ph.D. Candidate and Project Manager at Sinai BioDesign and Dr. Christopher Kellner, Assistant Professor and Director of the Intracerebral Hemorrhage Program were chosen from a highly competitive pool of applicants to participate in the JHU-Tufts 2020 Design Lab. The Design Lab, jointly run by the Tufts Institute for Clinical Research and Health Policy Studies, the MIT Center for Biomedical Innovation, and the CTSA Trial Innovation Network, explores innovations in clinical trial design in the context of the full treatment development pathway, with a particular focus on innovative clinical effectiveness trial design.
Since then, the Mount Sinai team has recurringly worked with the Design Lab consultation team to perfect their proposal. The goal of the JHU-Tufts Design Lab, in partnership with MIT, is to hone and promote paradigm-shifting ideas by bringing patients, payers, regulators, healthcare providers, and other key stakeholders together for an extended, interactive discussion. Emphasis is placed on new trial designs that bridge the divide between efficacy and effectiveness.
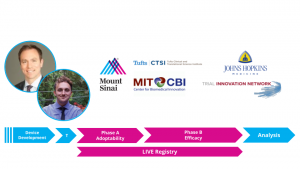
This month they present at the official Design Lab event, where they will introduce their novel trial design to a panel of 40+ experts in the fields of clinical trials, surgery, and statistics, as well as members of the FDA and medical device industry. Turner Baker and Dr. Christopher Kellner, both members of Mount Sinai’s Department of Neurosurgery, conceived of the novel design through their collaborative work on a new medical technology they are preparing for upcoming trials. Their ‘Live, Interactive Virtual Education (LIVE) Registry’ trial structure utilizes modern video sharing technology and a unique design to incorporate device training into surgical trials. An excerpt from their briefing book can be seen below:
The Problem.
Unlike pharmacological interventions, the clinical outcomes of surgery are highly correlated to the skill of the primary surgeon and associated team. The stated goal of randomized controlled trials (RCTs) is to exert control across all potential confounders to ascertain the effect of only the novel element in question. Stated another way, an RCT is designed to determine and measure efficacy. To assess the quality of new pharmacological therapeutics, closely monitored trials assessing optimal dose are conducted before capturing efficacy data. Surgical trials do not have this step, as administering only a fraction of a procedure or using only a piece of a device is often not feasible. Surgical trials, therefore, move directly into assessing efficacy. This is a mistake and is leading to false-negative trials. Surgical trial design must account for surgical skill when assessing the efficacy of novel devices and techniques, requiring participating surgeons to reach a minimum ‘surgical proficiency’ before clinical usefulness can be measured.
The Solution.
The Live, Interactive Virtual Education (LIVE) Registry trial design is a new adaptive trial design for evaluating the clinical performance of novel surgical techniques and devices. The LIVE trial design is broken into two distinct phases: Phase A, “Adoptability” and Phase B, “Efficacy.” This adoptability-to-efficacy (A2E) approach allows for discrete assessment between surgical skill development and intervention efficacy. The LIVE trial design also integrates modern live-streaming video services to increase surgeon exposure to cases and enable expert-to-expert learning. The LIVE trial design provides two major improvements over modern surgical technique/device trials: minimizing bias introduced through surgical inexperience, and reducing the time for surgeons to reach ‘surgical proficiency’.”
Applications for the next round of The Design Lab will be available in early 2021.
ConduITS is supported by NCATS of the NIH’s CTSA Program. Any use of CTSA-supported resources requires citation of grant number UL1TR001433 awarded to ISMMS in the acknowledgment section of every publication resulting from this support. Adherence to the NIH Public Access Policy is also required.


