ATTENTION
RUTH, the new IRB electronic submission and application tracking and review software rolled out on Wednesday, 9/23/20. Please go to the Research Roadmap RUTH – IRB Submission System page for more information and/or contact PPHS if you have any questions IRB@mssm.edu (212) 824-8200.
RUTH Training Sessions
IRB Virtual Office Hours
Mondays from 10:00 – 11:00 am
Wednesdays from 2:00 – 3:00 pm
Learn More
Research ListServ
Please sign up to Research Listserv, the research administration email messaging system to receive the latest announcements below and information including RUTH updates, funding opportunities, policy updates and more.
Clinical Research Forum (CRForum)
The Forum has been created to facilitate ongoing communication between research personnel like you and the administrative offices that regulate and process research projects (e.g., IRB, IACUC, GCO).
Held on the first Wednesday of each month, 12:00 – 1:00 p.m.
Register here
Past recordings found on the here
February and October reserved for IACUC Forums
Clinical Research Forum - Wednesday, November 5, 2025 - 11/5/2024
Please join us TODAY! Wednesday November 5, 2025, from 12:00 to 1:00 p.m. for the Clinical Research Forum (CR Forum). You must register for the Forum – please see the Zoom registration link below.
AGENDA
Welcome – Megan Richmond, CIP, Assistant IRB Manager, Program for the Protection of Human Subjects (PPHS)
Drug-Eluting Stents: What’s Required When Submitting To The IRB When Using These Stents – Dr. Glenn Martin, Senior Associate Dean for Human Subjects Research, Program for the Protection of Human Subjects (PPHS)
Updated MRI Research Guidelines – Christopher Cannistraci, MS, Director, Research Operations: BioMedical Engineering and Imaging Institute
Questions/Concerns/Open Mic
Register in advance for this webinar: Click here
Effective September 10, 2025, New IRB Guidance Document Now Available and Updates to RUTH - 9/10/2024
PPHS is pleased to announce the release of our latest guidance document, titled “HRP-937 – Guidance – Approving and Stamping IRB Documents in RUTH”. This resource lays out which study documents are finalized and which receive the IRB approval stamp.
Our goal with this guidance is to bring more clarity and consistency to documentation stamping practices. This guidance explains the difference between finalized vs. stamped documents and outlines exactly which types of documents will be stamped upon IRB approval. For example, the protocol, consent forms, recruitment materials, HRP-503 applications, and Research Information Sheets/Standalone HIPAA will continue to receive the IRB stamp upon approval. Whereas, Data Safety Monitoring Plans, Data Safety Monitoring Board Charters, device attachment(s), and drug attachment(s) will no longer be stamped once the project is approved.
This new guidance will be posted in the RUTH library under the General tab, and on the PPHS website under the Guidance and Policies page.
PPHS would also like to announce another small but important update in RUTH: a new document category called “Study participant-facing, non-recruitment” has been added. This new label should be used when uploading post-consent participant materials (e.g., participant diaries, study wallet cards) to the RUTH Smartform. Please note that study materials seen by or given to participants after they were consented to participate will not be stamped. This update goes live in RUTH on September 10, 2025.
If there are any specific questions, please send them to IRB@mssm.edu.
New IRB Fee Schedule Effective October 1, 2025 - 9/4/2024
Effective October 1, 2025, the Program for the Protection of Human Subjects (PPHS) will implement an updated fee schedule for all industry-sponsored human subjects research projects submitted for review.
PPHS has not adjusted IRB-related fees since 2019. However, with increasing costs tied to regulatory compliance, operational support, and the growing administrative complexity of industry-sponsored and externally funded research, a revised fee structure will take effect on October 1, 2025. As part of this process, we reviewed the posted rates of our regional peers as well as those of comparable academic institutions across the country. The revised structure reflects the services, expertise, and institutional resources required to support high-quality research and to ensure compliance with evolving regulatory standards.
As part of this change, PPHS will no longer invoice industry sponsors directly. Instead, researchers will be required to provide an unrestricted fund number at the time of their submission in RUTH and will be billed once IRB approval has been granted. Additional details are outlined in the attached document.
Clinical Research Forum - Wednesday, September 3 2025 - 8/26/2024
Please join us – September 3, 2025, from 12:00 to 1:00 p.m. for the Clinical Research Forum (CRForum). You must register for the Forum – please see the Zoom registration link below.
AGENDA
- Welcome
Lori Jennex, Associate Dean, Program for the Protection of Human Subjects (PPHS) - Chart Review Submissions: Tips and Tricks for a Streamlined Review Process: Sasha Broadstone, Analyst II, Program for the Protection of Human Subjects (PPHS)
- Updates on Ads, CVs, HIPAA Waivers for Screening, Representative Samples of Community and Progress Reports: Dr. Glenn Martin, Senior Associate Dean for Human Subjects Research, Program for the Protection of Human Subjects (PPHS)
- Questions/Concerns/Open Mic
Register in advance for this webinar: Click here
Clinical Research Forum Cancelled - Wednesday, August 6, 2025 - 7/30/2024
The Clinical Research Forum on Wednesday August 6, 2025 is cancelled. The next one will be held on Wednesday September 3, at 12pm. We hope you will be able to join us.
Enjoy the rest of the Summer!
Clinical Research Forum - TODAY - Wednesday, July 9, 2025 - 7/9/2024
Please join us, TODAY – Wednesday, July 9, 2025, from 12:00 to 1:00 p.m. for the Clinical Research Forum (CRForum). You must register for the Forum – please see the Zoom registration link below.
AGENDA
•Welcome
Lori Jennex, Associate Dean, Program for the Protection of Human Subjects (PPHS)
•Payment Requests/Paying Human Subjects
William LaDue, Corporate Director of Accounts Payable, MSHS This is your opportunity to have access to those who have the answers to your questions related to paying human subjects from Sinai Cloud. Whether its about processing payments or addressing Cloud related technical issues, this open Forum is where you can ask all your questions and get answers from the experts.
•Workflow Changes to Ordering Imaging – BMEII
Christopher Cannistraci, Director, BMEII
Register in advance for this webinar: Click here
Clinical Research Forum - Date Change to July 9, 2025 - 6/25/2024
The Clinical Research Forum (CRForum) scheduled for Wednesday, July 2, 2025 at Noon has been rescheduled due to the 4th of July holiday. Instead, the July CRForum will be held on July 9, 2025 at Noon. DTP/Sinai Cloud experts and a Finance team are coming together to answer questions on the process to pay human subject participants for time/effort and reimbursement when enrolled in a research study.
If you have any questions you’d like to send in advance, please email the IRB@mssm.edu.
You must register for the CRForum here.
Happy 4th of July!
Clinical Research Forum - TODAY - June 4, 2025 - 6/4/2024
Please join us, TODAY, June 4, 2025, from 12:00 to 1:00 p.m. for the Clinical Research Forum (CRForum) You must register for the Forum – please see the Zoom registration link below.
AGENDA
•Welcome
Lori Jennex, Associate Dean, Program for the Protection of Human Subjects (PPHS)
•Harnessing Epic Research: Features and Functionality
Parina Shah, Senior Director, Office of Research Services (ORS)
Jackie Dosik, Epic Research Program Manager, Office of Research Services (ORS)
Gabby Krol, Product Owner, Epic Research, Digital and Technology Partners (DTP)
•OnCore Payments
Danielle Chaney, OnCore Manager, Office of Research Services (ORS)
•Questions/Concerns/Open Mic
Register in advance for this webinar: Click here
Ruth Abramson Lecture Series Presents: Health and Human Services Policy Changes and the IRB - 5/8/2025
The Program for the Protection of Human Subjects: Ruth Abramson Lecture Series
Presents: Conducting Research in Today’s Regulatory Environment
Lauren Hartsmith, JD, CIP
Please join your colleagues on May 28, 2025
The Mount Sinai Hospital | Davis Auditorium
11 am – 12 pm
Refreshments will be served
The event is also available via Zoom – Registration is required
About the Presenter:
Lauren Hartsmith is an administrative law and regulatory compliance attorney, and the Principal Consultant for Hartsmith Consulting, LLC. She has experience working with government, institutional, non-profit, and for-profit IRBs and HRPPs, and is excited to help evolve the research ethics/compliance field in this new capacity.
Lauren’s experience includes serving as a Policy Analyst/Senior Advisor for Public Health Education with the Office for Human Research Protections (OHRP) where she led scientific, regulatory, and legal experts to develop and revise policies and regulations. During her tenure at OHRP, she was a key analyst involved in all aspects of the revised Common Rule rulemaking process. Lauren has given over 150 trainings on research requirements such as the Common Rule, FDA regulations, and Good Clinical Practice guidelines.
Most recently, Lauren was the Director of IRB Support and Regulatory Affairs at Advarra, where she managed a variety of functions including oversight/development of IRB guidance and policies, IRB member education, IRB meeting minutes, and written reports to regulatory authorities.
Clinical Research Forum - Wednesday, May 7, 2025 - 5/5/2025
Please join us for the Clinical Research Forum (CRForum) on May 7, 2025 from 12:00 to 1:00 p.m. You must register for the Forum – please see the Zoom registration link below.
AGENDA
•Welcome
– Lori Jennex, Associate Dean, Program for the Protection of Human Subjects (PPHS)
•Labor and Delivery Research – How Can We Best Support Patients?
– Meghan Proehl, MSW, MPH, Director, Research Administration, Department of Obstetrics, Gynecology, and Reproductive Science
•Questions/Concerns/Open Mic
Register in advance for this webinar: Click here
Clinical Research Forum - TODAY - Wednesday, April 2, 2025 - 4/2/2024
Please join us, today, for the Clinical Research Forum (CRForum) – April 2, 2025 from 12:00 to 1:00 p.m. You must register for the Forum – please see the Zoom registration link below.
AGENDA
•Welcome
– Lori Jennex, Associate Dean, Program for the Protection of Human Subjects (PPHS)
•New Imaging Order Research Questions Outside BMEII
– Chris Cannistraci, BMEII Director, Research Operations
•Continuing the Discussion: Request to Rely (R2R) Misconceptions
– Lori Jennex, Associate Dean, Program for the Protection of Human Subjects, (PPHS)
•Questions/Concerns/Open Mic
Register in advance for this webinar: Click here
PPHS Guidance in Uncertain Times - 3/13/2024
In the face of continuing budgetary uncertainties, as well as demands for the removal of certain words, phrases, ideas and concepts from grants, protocols, and consents, the PPHS wishes to share the following principles:
1) All non-emergent changes must be approved by the IRB of Record before they can be implemented. The review of all changes will follow current regulations, policies and procedures. There have been no changes to the federal regulations governing IRBs.
2) When submitting a modification to the IRB there needs to be a clear rationale for the change. Any change that touches study design or the interpretation of data will additionally need an analysis of how the change impacts the data already collected and the ultimate validity of the project.
3) Given the increasing probability that some studies will have to wind down, we remind investigators of the ethical imperative to make sure that it is done in a safe manner. We urge our investigators to be proactive in this regard and to be forthright in discussions with sponsors about this obligation.
4) We will update this guidance as events dictate. Please contact the PPHS as needed for specific issues to your projects.
If you have any questions regarding this message, please write to the IRB Inbox (IRB@mssm.edu).
PPHS Announces New Policies and Guidance Effective February 1, 2025- 1/23/2024
On December 12, 2024, PPHS sent out requests for comments on two policy changes (protocol requirements and honest brokers) which were going into effect in 2025. We thank those who took the time to engage in the process.
We received comments about the protocol policy asking to define interventional studies, whether observational studies would be required to have standalone protocols and where to find a template protocol. For the honest broker guidance, we received a question about when honest brokers can be removed from a project and confirming those listed in RUTH as honest brokers are those who work at Mount Sinai. All the questions asked have been addressed in the new policies/guidance being released.
Effective February 1, 2025 PPHS is requiring the following changes to be implemented as appropriate:
1. Required Protocols: Per our correspondence on December 12, 2024, PPHS will require a standalone, complete protocol with the RUTH submission for all interventional protocols. For the purposes of this new requirement, interventional studies include any study where there is any interaction between the participant and the research team. For example, a prospective study which would include occasional clinical visits, questionnaires, focus groups and chart reviews, would be considered “interventional.” Clinical trials are certainly included, and retrospective chart reviews would not.
The NIH has a protocol template (found here) that can be used as a standalone protocol but other formats are acceptable as long as the information is included. An HRP-503 form is still required to be submitted with the protocol, however with a well written protocol, most of the questions can be answered by referring to the protocol. Please note, that the standalone protocol can point to the grant application for the background and science sections only.
2. The Honest Broker Guidance Form HRP-936 – This new guidance outlines the process for the use of the Honest Broker. Going forward PPHS expects the honest broker to be listed in the application as research personnel and they must be listed in RUTH with the role of Honest Broker on the Local Study Team Members tab (available in RUTH on 2/1/25). There needs to be a written description of the honest broker’s functions and limitations that should also be included in the RUTH application, either in the protocol/HRP-503 form or as a standalone document.
3. Consent Form Summary Requirements: The last modifications to the Common Rule promoted the use of a concise introductory summary in the consent form to highlight the important aspects of the research project and set the stage for the rest of the consent document. With the last update of the PPHS consent template in 2022 we reiterated the need to have consents written at an 8th grade reading level, with particular emphasis on the introduction section. To improve the readability and quality of research informed consent forms, the PPHS tested a new process to help study teams design a better summary. That process is now becoming a requirement for all projects (with the exception of Request to Rely (R2R) projects). The summary section of your consent form should not be more than 500 words, Flesch-Kincaid Reading Ease grade should be less than 70, and/or the Flesh-Kincaid grade level should be below a 9th grade level. A document will be posted in the RUTH library.
4. Checklist HRP-450 Required with all Initial submissions: PPHS will now require Checklist HRP-450 to be completed and uploaded with your submission in RUTH. Of note, the HRP-450 checklist has been updated to include a section regarding the readability of the summary section of the consent form. In all consent forms, the summary sections should not be more than 500 words, Flesch-Kincaid Reading Ease grade should be less than 70, and/or the Flesh-Kincaid grade level should be below a 9th grade level. See #3 above for more information on the new requirement for the consent summary section of a consent form. See the HRP-916 document in the RUTH library for R2R forms. Currently the readability requirement does not apply to R2R submissions.
5. HRP-902 Case Report Policy has been updated and can be found on the website with the other updated forms over the next few days.
Clinical Research Forum Cancelled - Wednesday, January 1, 2025- 12/30/2024
The Clinical Research Forum on Wednesday, January 1, 2025 is cancelled. Happy New Year!
PPHS Request for Comments on New Guidance- 12/12/2024
The PPHS is distributing two policy updates and requesting comments prior to implementation. Please review the following information and the linked document. We ask that you submit your comments to the IRB Inbox at IRB@mssm.edu, by Noon on December 19, 2024. Upon review, we may reach out to get more information.
1. Need for Protocols
The PPHS has accepted grant proposals in lieu of protocols for federally funded projects and some grants. Going forward, we will require a standalone, complete protocol with the RUTH submission for ANY AND all interventional protocols. By standalone, we mean that the protocol cannot reference other related documents (e.g., “see grant”) other than references to the scientific basis and background for the study. The protocol should be accompanied by the HRP-503, but that document can refer liberally to the protocol if the answers can be found there. We are doing this as several audits and reviews have concluded that the lack of a protocol has caused confusion among researchers and ambiguity in trying to demonstrate what the IRB approved. And of course, while the grant application is static, most projects evolve and modify over the years.
For now, this requirement is for initial submissions but the PPHS also believes it is necessary for a standalone protocol to be submitted for ongoing projects at the time of renewal or substantial modification, i.e., not a simple personnel modification. We recognize that this will be extra work for established projects, but our experience has shown that this is exactly the sort of project that has gotten into problems during external review. We plan on implementing this requirement for existing projects, a few weeks after the initial project roll-out has begun.
2. Honest Brokers Guidance
We are also distributing new guidance for the use of honest brokers. An honest broker can provide a firewall between an investigator and a study participant’s identifiable information. For example, an honest broker could generate or receive a dataset and then remove subject identifiers so that the data was no longer readily identifiable. This new guidance outlines the process for the use of the Honest Broker. Going forward PPHS expects the honest broker to be listed in the application as research personnel and they must be listed in RUTH with the role of Honest Broker on the Local Study Team Members tab. There needs to be a written description of the honest broker’s functions and limitations that should also be included in the RUTH application, either in the protocol/HRP-503 or as standalone document.
Click here to review the honest broker document.
We thank you in advance for your interest and suggestions. If you need further information, please contact the PPHS in the usual manner, phone 212-824-8200 or general email at IRB@mssm.edu.
PPHS Closed, TODAY - Tuesday, 12/10/24 from 1:00 pm to 5:00 pm- 12/10/2024
The PPHS will be closed TODAY at 1:00 PM for our staff retreat. We will re-open at 9:00 AM on Wednesday, December 11th. You’re welcome to leave a message on the main telephone line (212-824-8200) or email irb@mssm.edu with questions while we are away. If you have a human subject emergency during this time, please call Lori Jennex, Associate Dean, PPHS at 646-285-3689.
Clinical Research Forum - TODAY, December 4, 2024- 12/4/2024
Please join us for the Clinical Research Forum (CRForum) TODAY, December 4, 2024 from 12:00 to 1:00 p.m. You must register for the Forum – please see the Zoom registration link below.
AGENDA
•Welcome
– Lori Jennex, Associate Dean, Program for the Protection of Human Subjects (PPHS)
•New IRB Consent Requirements ~ January 2025
•New IRB Form Requirements ~ January 2025
•Future Use Consent Language and Genetic Testing Consent Language – What You Need to Know if You Use an External IRB!
– Glenn Martin, MD; Senior Associate Dean for Human Subjects Research, Executive Director, PPHS
•Questions/Concerns/Open Mic
Register in advance for this webinar: Click here
PPHS Closed on Friday, November 29th- 11/25/2024
The PPHS will be closed Thursday and Friday, November 28th and 29th for the Thanksgiving holiday. Please plan accordingly. You’re welcome to leave a message on the main telephone line 212-824-8200 or email irb@mssm.edu with questions while we are away. If you have an emergency on Friday 11/29/24, you can call Lori Jennex, Associate Dean, PPHS at 646-285-3689.
We will reopen on Monday, December 2nd at 9:00 am and will answer your inquires at that time.
Wishing you a safe & happy holiday,
Program for the Protection of Human Subjects
Forms Available in RUTH- 11/13/2024
As a reminder, PPHS provides valuable resources to support you with your IRB/RUTH applications.
You can find comprehensive guidance, worksheets, and checklists in the RUTH library and on the Program for the Protection of Human Subjects (PPHS) website.
The Table of Contents for all our resource offerings is located on the General tab in the RUTH library and is called HRP-100 – Huron HRPP Toolkit Table of Contents.
The PPHS encourages you to explore these resources to ensure a smooth submission process.
Clinical Research Forum - TODAY - November 6, 2024 - 11/6/2024
TODAY’s Reminder – November 6, 2024 – the Clinical Research Forum (CRForum) will be hosted by PPHS from 12:00 pm to 1:00 p.m.
You must register for the Forum – please see the Zoom registration link below.
AGENDA
- Welcome – Lori Jennex, Associate Dean, Program for the Protection of Human Subjects (PPHS)
•Office of Research Services (ORS): How the Office of Research Services can help in navigating research systems/Infrastructure of MSHS!: Parina Shah, Director, Samuel Pugo, Project Manager, Courtney Sheldon, Project Manager, Danielle Chaney, OnCore Manager•Newly Released Forms (HRP-212b, HRP-503R, HRP-935), Required Checklist (HRP-451) and Banking Data:Glenn Martin, MD; Senior Associate Dean for Human Subjects Research, Executive Director, PPHS•Questions/Concerns/Open Mic
Register in advance for this webinar: Click here
Effective October 28, 2024, PPHS Requires New and Updated Forms to be Used in RUTH Submissions - 10/25/2024
The PPHS is pleased to announce that the following forms and template have been added to the RUTH library and will be on the PPHS website soon:
• UPDATED: HRP-212B – Annual Activity Report for Continuing Review Specimen or Data Banking
• UPDATED: HRP-503R – Record/Specimen Review Application
• NEW: HRP-935 – Continuing Review Progress Report Instructions
Effective October 28, 2024, these updated/new documents must be used with your submissions. Additionally, the HRP-451- Continuing Review-Final Reports Checklist must be included with all continuing review submissions. Please note the PPHS will not begin reviewing continuing review applications without the completed HRP-451 form attached.
The updated HRP-212B must be used for all continuing review submissions that involve banking of specimens and/or data.
The updated HRP-503R template will need to be used for all new initial record review submissions.
The new guidance document, HRP-935 – Continuing Review Progress Report Instructions, serves to instruct researchers on the required information that must be included in the research progress report and submitted with each continuing review submission.
Click here to see a list of changes made to each document. The list of changes will be added to our website soon.
If there are any specific questions, please send them to IRB@mssm.edu.
Final Version HRP-928 Screening Procedures for Research - 8/5/2024
The updated HRP-928 Screening Procedures for Research is now finalized. Thank you for your feedback and comments. As a reminder, we made changes to this guidance document because the 2018 update to federal regulations allow for certain screening activities to be done without the need for consent or a waiver of consent. This, however, does not negate the need for HIPAA Authorization or a HIPAA waiver when Protected Heath Information (PHI) is involved in those screening activities.
Click here to review the final version of the HRP-928 Screening guidance. The updated guidance is active immediately.
Dr. Glenn Martin will be discussing the changes to the screening guidance at the Clinical Research Forum (CRForum) on Wednesday, August 7 (Register here to attend). If you have any questions or comments, bring them to the CRForum. If you are unable to attend the CRForum, please email IRB@mssm.edu with your questions and comments.
Thank you for your participation in this process and your ongoing commitment to ethical and compliant human subjects research.
Clinical Research Forum - August 7, 2024 - 8/5/2024
Please join us for the Clinical Research Forum (CRForum), Wednesday, August 7, 2024 from 12:00 to 1:00 p.m. You must register for the Forum – please see the Zoom registration link below.
AGENDA
Lori Jennex, Associate Dean, PPHS:
•Welcome
Daniel Lennard, Assistant General Counsel, Mount Sinai Legal Department:
•GDPR and International Data Issues
Dr. Glenn Martin, Senior Associate Dean for Human Subjects Research & Executive Director, PPHS:
•Announcing Updated Screening Policy
•Record Review(s) Update
•Consent Summary Improvement Project Update
•Questions/Concerns/Open Mic
Register for the webinar here.
UPDATED: Revised HRP-212B and HRP-503 Documents Effective September 2, 2024 - 7/18/2024
Further Information for Clarification: On or after the effective date of September 2, 2024, the requirement to use the updated HRP-212B will apply to all new continuing review submissions. The requirement to use the HRP-503 templates will apply to all new initial study submissions. Research teams will not be required to transfer to the updated HRP-503 templates.
The PPHS is pleased to announce that the following form and templates have been updated on the PPHS website and in the RUTH library:
- HRP-212B Continuing Review Specimen Banking Progress Report
- HRP-503 – Full Version
- HRP-503R – Record/Specimen Review Application
- HRP-503E – Exempt Determination Application
The effective date for these updated documents is September 2, 2024. This effective date is when use of these documents will be required, however, you can begin to use them now.
Click here to see a list of changes made to each document. The list of changes will be added to our website soon.
If there are any specific questions, please send them to IRB@mssm.edu
Revised HRP-212B and HRP-503 Documents Effective September 2, 2024 - 7/18/2024
The PPHS is pleased to announce that the following form and templates have been updated on the PPHS website and in the RUTH library:
- HRP-212B Continuing Review Specimen Banking Progress Report
- HRP-503 – Full Version
- HRP-503R – Record/Specimen Review Application
- HRP-503E – Exempt Determination Application
The effective date for these updated documents is September 2, 2024. This effective date is when use of these documents will be required, however, you can begin to use them now.
Click here to see a list of changes made to each document. The list of changes will be added to our website soon.
If there are any specific questions, please send them to IRB@mssm.edu.
Volunteers Needed for Consent Form Summary Improvement Testing - 7/17/2024
Research informed consent forms should provide detailed accessible information to potential research participants. For consent to be fully informed, in addition to containing specific details, the consent form must be readable and comprehensible. The last modifications to the common rule promote the use of a concise introductory summary in the consent to highlight the important aspects of the research project and set the stage for the rest of the consent. With the last update of the PPHS consent template in 2022 we reiterated the need to have consents written at an 8th grade reading level, with particular emphasis on the introduction section. While there has certainly been progress, consents are still being submitted for approval which are unacceptably complex.
To improve the readability and quality of research informed consent forms, the PPHS is testing a new process to help study teams with their consent form design before the application is submitted for IRB review. We are adding some objective scoring tools found within Microsoft Word, to the process, which should allow for teams to self-identify those consents that need to be improved. This testing and potential implementation does not apply to R2R projects.
We are seeking volunteers who are submitting initial studies that include consent forms in RUTH between July 29 – Aug 2. To support our new process testing, we are seeking 10 – 12 initial study submissions. If there are no/ insufficient number of volunteers, we will identify initial submissions that come in between July 29 – Aug 2 to participate in this testing.
- Beginning July 29, 2024, selected submissions will be required to provide a word count of the Summary section not to exceed 500 words and an 8th grade level for readability.
- Using Word’s Editor/Insights feature (document stats), calculate the word count and readability statistics for the project’s consent form summary section.
- With your application submission provide a copy of the determination which will include the word count of the Summary section (example provided below).
- All consent form summary sections included in your application that present higher than 500 words, a Flesch-Kincaid Reading Ease grade of 70 or higher and a Flesh-Kincaid grade level of 9th or above will not be accepted and will be sent back to you for editing.
We will provide guidance on the above process, as needed, for study teams involved in this testing.
If you need help with re-wording the consent summary section, you can use tools like Microsoft Copilot for AI-generated suggestions on how to improve the readability.
Please email irb@mssm.edu by Tuesday, July 23, if you are interested in participating in this pilot test. Thank you for your anticipated participation
Request for Comments: HRP-928 - Screening Procedures for Research - 7/15/2024
The Program for the Protection of Human Subjects (PPHS) has updated the HRP-928 Screening Procedures for Research Guidelines. The 2018 update to the federal regulations governing human subjects research allow for certain screening activities to be done without the need for consent or a waiver of consent. This, however, does not negate the need for HIPAA authorization or a HIPAA waiver when PHI is involved in those screening activities.
Today, we would like to distribute the updated guidelines for comment by you, the ISMMS research community. We have made updates throughout the document to reflect the above regulatory change and ask that you review the document and share with us whatever suggestions you might have. Your comments can focus on the content, the instructions, or whatever you think will be helpful in improving the document. We ask that you submit your comments within the next 2 weeks to IRB@mssm.edu, with all comments due by Friday, July 26 at the close of business. Upon review, we may reach out to get more information.
Click here to review the document.
We thank you in advance for your interest and suggestions. If you need further information please contact the PPHS in the usual manner, phone 212-824-8200 or general email at IRB@mssm.edu
CRForum - Cancelled - July 3, 2024 - 7/1/2024
The Clinical Research Forum (CRForum) scheduled for Wednesday, July 3, 2024 at Noon is cancelled. If you have any questions, please send them to IRB@mssm.edu.
Happy 4th of July!
HIPAA Wizard Request for Review by Research Community - 7/1/2024
Two years ago, The Program for the Protection of Human Subjects (PPHS) updated the HIPAA Wizard to the current version used in RUTH today. In that time, we have received feedback on how to make the wizard clearer, and in turn, more helpful to the research community.
We are seeking additional feedback on the HIPAA Wizard before we make new updates/ changes. Email IRB@mssm.edu to provide feedback on any aspect of the HIPAA Wizard that you think can be improved on. As a reminder, the goal of the HIPAA Wizard is to provide guidance on the appropriate HIPAA forms to be completed and submitted as part of a study’s RUTH application. We ask that you submit your comments/ suggestions within the next 2 weeks, with all comments due by Friday, July 12 at the close of business. Upon review, we may reach out to get more information.
Click here to find the current version of the HIPAA Wizard.
We thank you in advance for your interest and suggestions. If you need further information please contact the PPHS in the usual manner, phone 212-824-8200 or general email at IRB@mssm.edu
Clinical Research Forum - June 5, 2024 - 6/3/2024
Please join us for the Clinical Research Forum (CRForum), TODAY, 6/5 from 12:00 to 1:00 p.m. You must register for the Forum – please see the Zoom registration link below.
AGENDA
•Welcome, Marilyn Eshikena, Assistant Director, PPHS
•Radiation Safety Review Process: Ilda Bander, MD; Clinical Research Manager, Department of Diagnostic, Molecular and Interventional Radiology
•ClinicalTrials.gov: FAQ and maybe not so FAQ; Samuel Pugo, Project Manager: Litzzy Lugo, Research Coordinator: Angela Lee, Project Manager II
•Questions/Concerns/Open Mic
Register for the webinar here.
CITIProgram Human Subjects Certificate Information Now Available in Sinai Central- 5/15/2024
CitiProgram education certificate reports, for all departments, are accessible now in Sinai Central. Now you can easily know which researchers are up-to-date on their CitiProgram education requirements.
▶ Department Administrators have access to CITI research certifications in Sinai Central. The report includes all certification data for faculty and research staff who have indicated an affiliation with the Icahn School of Medicine at Mount Sinai.
▶ People assigned the Sinai Central role of Department Administrator for any department are automatically granted access to the report at the location above.
▶ Department Administrator assignees for any department also can grant view access to the report to non-Department Administrators by assigning them the department-level role DEPT CERTIFI VIEW.
▶ For instructions follow the link here.
Closed Friday, May 3, 2024 for IRB Education Retreat- 4/29/2024
PPHS will be closed Friday, May 3, 2024 for our IRB Education Retreat. Please plan accordingly. You’re welcome to leave a message on the main telephone line 212-824-8200 or email irb@mssm.edu with questions while we are away. If you have an emergency, you can text or call Lori Jennex, Associate Dean, PPHS at 646-285-3689.
We will reopen on Monday, May 6th at 9 am and will answer your inquires at that time.
Final Version HRP-927 Use of Drawings in Research Guidance- 4/25/2024
The PPHS has developed new guidance on the use of drawings within research projects to incentivize participant engagement.
The new guidance covers the NYC metropolitan area, the tri-state area and Florida which should simplify using a drawing in larger projects.
Click here to review the HRP-927 guidance.
We intend to put the guidance into action on May 1, 2024 and we wanted to give some time for feedback from the research community to make any necessary adjustments. If you choose to comment on the guidance or you have specific questions, please send them to IRB@mssm.edu.
Thank you for your participation in this process and your ongoing commitment to ethical and compliant, human subjects research.
PPHS and Tisch Cancer Institute Announce PRMC Becomes an Ancillary Office in RUTH- 3/5/2024
PPHS and the Tisch Cancer Center would like to announce the Protocol Review and Monitoring Committee (PRMC) will become an Ancillary Office beginning Monday, March 11, 2024 when the upgrade to the RUTH software platform is finalized.
Information will be discussed at the Clinical Research Forum (CRForum) on Wednesday, March 6th from Noon – 1:00 pm. Currently the use the of the PRMC Ancillary Office process will be limited to initial submissions when using concurrent review for Industry sponsored clinical trials.
Please join us at the CRForum (register here) to learn more about this new change.
Thank you,
Therica MillerExecutive Director, Enterprise Cancer Clinical ResearchThe Tisch Cancer InstituteAssociate Dean, Cancer ProgramsIcahn School of Medicine at Mount Sinai
Lori JennexAssociate DeanProgram for the Protection of Human SubjectsIcahn School of Medicine at Mount Sinai
Clinical Research Forum - March 6, 2024- 3/5/2024
Please join us for the Clinical Research Forum (CRForum) tomorrow, Wednesday, March 6, 2024 from 12:00 to 1:00 p.m. You must register for the Forum – please see the Zoom registration link below.
AGENDA
•Welcome, Lori Jennex, Associate Dean, PPHSGoogle Voice and Virtual Office Hours
•PRMC will be a new Ancillary Office – Keri Strauss, PRMC
•New Process for Initial Reviews with IDS and PRMC – Jennifer Kucera, Associate Director, Regulatory Affairs•Brand Overview for Research Associates: Ron Sohn, Branding Specialist, Mount Sinai Department of Marketing & Communications•How to keep information on people who don’t want to be contacted for research and There is no such thing as a “partial HIPAA waiver”: Dr. Glenn Martin, Senior Associate Dean for Human Subjects Research•Questions/Concerns/Open Mic
Register in advance for this webinar here.
PPHS Re-accreditation Site Visit Next Week- 2/20/2024
Next week the PPHS is hosting a re-accreditation site visit with the Association for the Accreditation of Human Research Protection Programs (AAHRPP). During this time you may experience some delay in responses from our team. Our office will be open all week, and we are available for assistance, but we appreciate your consideration and patience during the site visit days (2/28-2/29). For assistance during this time, we recommend contacting an analyst or staff member directly by calling their direct line or emailing them rather than calling the general telephone line. You can find our information here: Meet the Team.
For general questions, you may call 212-824-8200 or email irb@mssm.edu and we will be checking messages regularly and will respond as soon as possible.
Thank you for your understanding during this important visit for our program.
CRForum - January 3, 2024- 1/3/2024
Please join us for the Clinical Research Forum (CRForum) on Wednesday, January 3, 2024 from 12:00 to 1:00 p.m. You must register for the Forum – please see the Zoom registration link below.
AGENDA
•Welcome – Lori Jennex, Associate Dean, Program for the Protection of Human Subjects (PPHS)
•EPIC Research Module Overview AND Process Change in FACTS managed Industry Funded Clinical Trials effective Jan 1, 2024 – Office of Research Services (ORS), Parina Shah, Director
•Questions/Concerns/Open Mic
Register in advance for this webinar:https://mssm.zoom.us/webinar/r…
*Reminder – February 7, 2024 is the Basic Science Edition of the CRForum
PPHS Office Hours Cancelled - Wednesday, December 27, 2023- 12/27/2023
PPHS office hours are cancelled today, Wednesday, 12/27/23 from 2:00 pm – 3:00 pm.
Join us next Wednesday, 1/3/24 from 2:00 pm – 3:00 pm for help with your human subjects research questions. Use this Zoom link to join!
PPHS Closed the Afternoon of Wednesday, 12/13/23- 12/11/2023
The PPHS will be closed at 12:30 PM on Wednesday, December 13th for our staff retreat. We will re-open at 9:00 AM on Thursday, December 14th. You’re welcome to leave a message on the main telephone line (212-824-8200) or email irb@mssm.edu with questions while we are away. If you have an emergency during this time, please call Lori Jennex, Associate Dean, PPHS at 646-285-3689.PPHS Office Hours for 12/13/23 are cancelled.
CRForum - Cancelled - December 6, 2023- 12/1/2023
The Clinical Research Forum (CRForum) scheduled for Wednesday, December 6, 2023 at Noon is cancelled. If you have any questions, please send them to IRB@mssm.edu.
The CRForum on Wednesday, January 3, 2024 will host the Office of Research Services (ORS) whose team will speak on the topic of research billing in EPIC as it applies to human subjects research.
Happy holidays!
Friday, November 3, 2023 - PPHS Will Be Open- 11/3/2023
The IRB Education Retreat scheduled for this Friday, November 3, 2023 has been cancelled, therefore the PPHS will be open tomorrow.
Clinical Research Forum - November 1, 2023- 10/31/2023
Please join us for the Clinical Research Forum (CRForum) on Wednesday, November 1, 2023 from 12:00 to 1:00 p.m. You must register for the Forum – please see the Zoom registration link below.
AGENDA
•Welcome and Reminders – Lori Jennex, Associate Dean, PPHS•FCOI Ancillary Review Process Reminders & Tips– David Denhoff, Director, Industry Engagement and Conflicts of Interest, Office of the Dean, ISMMS•Questions/Concerns/Open Mic
Register in advance for this webinar:https://mssm.zoom.us/webinar/r…
Closed Friday, November 3, 2023 for IRB Education Retreat- 10/30/2023
PPHS will be closed Friday, November 3, 2023 for their IRB Education Retreat. Please plan accordingly. You’re welcome to leave a message on the main telephone line 212-824-8200 or email irb@mssm.edu with questions while we are away. If you have an emergency, you can call Lori Jennex, Associate Dean, PPHS at 646-285-3689.
We will reopen on Monday, November 6th at 9 am and will answer your inquires at that time.
Final Version HRP-921 - 2022 Informed Consent Form (ICF) Template Transition Guidance- 9/21/2023
PPHS is pleased to announce that after posting our revised HRP-921 2022 Informed Consent Form (ICF) Template Transition guidance for public comment, we have now finalized the document. Within the guidance you will find more detailed information on expectations of re-consenting and notification when using the 2022 consent template.
If there are any specific questions, please send them to IRB@mssm.edu.
If you are interested in setting up an individualized plan, which this guidance does allow, please discuss with PPHS prior to submitting a modification.
Thank you for your participation in this process and your ongoing commitment to ethical and compliant, human subjects research.
Click here to download the HRP-921 guidance.
NIH Grant Deadline - October 5, 2023 - Single IRB (sIRB) Requirements for Grant Submissions- 9/6/2023
For all competitive federal grant submissions (i.e., new, competitive renewal, or resubmissions) that are multi-site or collaborative, the use of a single IRB (sIRB) may be required by Federal Regulation.
- If you would like ISMMS to consider serving as the sIRB for your project, P.I.s must consult with the PPHS regarding 1) our ability to serve as the sIRB, and 2) sIRB fees to include in the grant budget.
- Please fill out the HRP-230 form and email to irb@mssm.edu for consideration at least two weeks prior to grant submission.
- If the ISMMS IRB agrees to serve as the sIRB, PPHS staff will provide sIRB fees to include in the grant budget and a letter of support from the PPHS Executive Director.
- Additional information is available here.
- If you would like to rely on an external IRB/institutional IRB other than ISMMS for a federally funded project, please consider the following:
- ISMMS IRB agrees to cooperate with the sIRB plan when the research is not a first in human trial or emergency research and the sIRB is one of the following:
- Accredited and utilizing the SmartIRB Master Common Reciprocal Institutional Review Board Authorization Agreement (SmartIRB Agreement), or
- A non-accredited CTSA hub with no OHRP/FDA warning letters utilizing the SmartIRB Agreement
- An external IRB with which ISMMS already has an existing master agreement (WCG, BRANY, Advarra, Alpha, NCI, NMDP)
- Additional information is available here.
- ISMMS IRB agrees to cooperate with the sIRB plan when the research is not a first in human trial or emergency research and the sIRB is one of the following:
Clinical Research Forum - September 6, 2023- 9/5/2023
Please join us for the Clinical Research Forum (CRForum) on Wednesday, September 6, 2023 from 12:00 to 1:00 p.m. You must register to attend the Forum – please use the Zoom registration link here.
AGENDA
•Welcome, PPHS
•How the Office of Research Services (ORS) can help in navigating research systems/Infrastructure of MSHS!
•Questions/Concerns/Open Mic
EXTENDED - Request for Comments Deadline - HRP-921 - 2022 Informed Consent Form (ICF) Template Transition Guidance Request for Review by Research Community- 8/21/2023
Request for comments has been extended to Thursday, August 24, 2023 at the close of the business day. Please see below.
Greetings:
The Program for the Protection of Human Subjects (PPHS) has completed its review of HRP-921 2022 Informed Consent Form (ICF) Template Transition guidance. The guidance (Sections I-III) has been in effect since December, 2022 and updated to reflect where we are in the transition. Research teams have asked for further clarification and modifications and thus we have worked to update the guidance to further address these requests. Much of the focus has been on 1) when and if consent forms need to transition to the latest 11.11.2022 version and 2) the requirement for notification. In the near future, PPHS will be providing a notification summary document which the research community may use if notification is part of their updated plan.
Today, we would like to distribute the updated version for comment by you, the ISMMS research community. We have made minor updates to the first parts of the document and ask that you review Sections IV and V (highlighted) of the document and share with us whatever suggestions you might have. Your comments can focus on the content, the instructions, or whatever you think will be helpful in improving Sections IV and V of the document. You may keep your identity anonymous, if you prefer. Upon review, we may reach out to get more information, assuming you identify yourself on the comment form.
Click here to find the form and the comments page.
We thank you in advance for your interest and suggestions. If you need further information please contact the PPHS in the usual manner, phone 212-824-8200 or general email at IRB@mssm.edu
HRP-921 - 2022 Informed Consent Form (ICF) Template Transition Guidance Request for Review by Research Community- 8/4/2023
The Program for the Protection of Human Subjects (PPHS) has completed its review of HRP-921 2022 Informed Consent Form (ICF) Template Transition guidance. The guidance (Sections I-III) has been in effect since December, 2022 and updated to reflect where we are in the transition. Research teams have asked for further clarification and modifications and thus we have worked to update the guidance to further address these requests. Much of the focus has been on 1) when and if consent forms need to transition to the latest 11.11.2022 version and 2) the requirement for notification. In the near future, PPHS will be providing a notification summary document which the research community may use if notification is part of their updated plan.
Today, we would like to distribute the updated version for comment by you, the ISMMS research community. We have made minor updates to the first parts of the document and ask that you review Sections IV and V (highlighted) of the document and share with us whatever suggestions you might have. Your comments can focus on the content, the instructions, or whatever you think will be helpful in improving Sections IV and V of the document. You may keep your identity anonymous, if you prefer. We ask that you submit your comments within the next 2 weeks, with all comments due by Friday, August 18 at the close of business. Upon review, we may reach out to get more information, assuming you identify yourself on the comment form.
Click here to find the form and the comments page.
We thank you in advance for your interest and suggestions. If you need further information please contact the PPHS in the usual manner, phone 212-824-8200 or general email at IRB@mssm.edu
Clinical Research Forum - Cancelled - August 2, 2023- 7/19/2023
The Clinical Research Forum (CRForum) scheduled for Wednesday, August 2, 2023 at Noon is cancelled. If you have any questions, please send them to IRB@mssm.edu.
We’ll look forward to seeing you on Wednesday, September 6, 2023.
Revised Reportable New Information (RNI) Reporting Guidance Effective September 1, 2023- 7/19/2023
The PPHS is pleased to announce that after posting our revised HRP-922-Guidance for Reportable New Information (RNI) Reporting for review and comments, the HRP-922 is now finalized. In addition, the companion HRP-923-Guidance for RNI Reporting for R2Rs has also been updated. The effective date for these updated guidance documents is September 1, 2023.
For studies under the ISMMS IRB, we recognize that many current projects may not have been submitting all RNIs consistent with this revised guidance, e.g. outside monitor reports and aggregate reports. Our expectation is that going forward, projects will become compliant with the new requirements. After September 1, 2023, all events that require prompt reporting under the new guidance are expected to be submitted in a timely manner. After September 1st, at the time of any project’s next continuing review, we expect that we will receive an aggregate report that will be complete, and will include all reportable events since the previous continuing review, whether they were reported properly or not prior to September 1st.
If there are any specific questions, please send them to IRB@mssm.edu.
If you are interested in setting up an individualized QA and reporting plan, which this guidance does allow, please discuss with PPHS prior to submitting a modification.
Thank you for your participation in this process and your ongoing commitment to ethical, compliant, human subjects research.
Click here to download the HRP-922 guidanceClick here to download the HRP-923 guidance
Clinical Research Forum July 5, 2023- 6/29/2023
Please join us for the Clinical Research Forum (CRForum) on Wednesday, July 5, 2023 from 12:00 to 1:00 p.m. You must register to attend the Forum – please use the Zoom registration link here.
AGENDA
•Welcome, Lori Jennex, Senior Director, PPHS
•ClinicalTrials.gov – Hopefully Helpful Hints: Samuel Pugo, Project Manager, Office of Research Services, ConduITS, Institutes for Translational Sciences
•GCO Resources and Training: Allison Gottlieb, Director, Sponsored Programs Education and Communications, Grants and Contracts Office
•Questions/Concerns/Open Mic
Due - Thursday June 29th - HRP-922 Reportable New Information Guidance - Request for Review by Research Community- 6/27/2023
Thank you to everyone who has submitted comments on the updated HRP-922 Reportable New Information (RNI) guidance. This is a reminder, for anyone who still wishes to submit comments, the deadline is Thursday, June 29, 2023, 5:00 p.m. Please see the information below and the link to the guidance and form you can use to submit your comments.
Greetings:
The Program for the Protection of Human Subjects (PPHS) has completed its review of HRP-922 Reportable New Information (RNI) guidance. The guidance has been in effect since 2010, recently clarified in 2021 for projects relying on external IRBs and in 2022 for projects relying on ISMMS IRBs. Research teams have asked for further clarification and modifications and thus we have worked to update the guidance to further address these requests. Much of the focus has been on clarifying prompt vs. annual aggregate reporting to the PPHS, and an emphasis on the need for the ongoing monitoring of projects by the PI, departmental regulator/quality programs and external monitors.
Today, we would like to distribute the updated version for comment by you, the ISMMS research community. We ask that you review the document and share with us whatever suggestions you might have. Your comments can focus on the content, the instructions, or whatever you think will be helpful in improving the document. You may keep your identity anonymous, if you prefer. We ask that you submit your comments within the next 2 weeks, with all comments due by Thursday, June 29th at the close of business. Upon review, we may reach out to get more information, assuming you identify yourself on the comment form.
Click here to access and review the updated guidance
We thank you in advance for your interest and suggestions. If you need further information please contact the PPHS in the usual manner, phone 212-824-8200 or general email at IRB@mssm.edu
RUTH Library Documents Refreshed!- 6/26/2023
Many documents in the RUTH Library have been refreshed to ensure the materials meet certain accessibility guidelines outlined in U.S. General Services Administration (GSA) section 508.gov and align with Microsoft Word’s “Check Accessibility” feature.
- Submissions in progress do not need to use the new forms. However, please use the current documents from the RUTH Library in future submissions.
- The HRP-503s have not changed at this time.
If you have any questions, please reach out to the PPHS at irb@mssm.edu
HRP-922 Reportable New Information Guidance - Request for Review by Research Community 6/15/2023
The Program for the Protection of Human Subjects (PPHS) has completed its review of HRP-922 Reportable New Information (RNI) guidance. The guidance has been in effect since 2010, recently clarified in 2021 for projects relying on external IRBs and in 2022 for projects relying on ISMMS IRBs. Research teams have asked for further clarification and modifications and thus we have worked to update the guidance to further address these requests. Much of the focus has been on clarifying prompt vs. annual aggregate reporting to the PPHS, and an emphasis on the need for the ongoing monitoring of projects by the PI, departmental regulator/quality programs and external monitors.
Today, we would like to distribute the updated version for comment by you, the ISMMS research community. We ask that you review the document and share with us whatever suggestions you might have. Your comments can focus on the content, the instructions, or whatever you think will be helpful in improving the document. You may keep your identity anonymous, if you prefer. We ask that you submit your comments within the next 2 weeks, with all comments due by Thursday, June 29th at the close of business. Upon review, we may reach out to get more information, assuming you identify yourself on the comment form.
Click here to access and review the updated guidance
We thank you in advance for your interest and suggestions. If you need further information please contact the PPHS in the usual manner, phone 212-824-8200 or general email at IRB@mssm.edu
Clinical Research Forum - June 7, 2023 6/5/2023
Please join us for the Clinical Research Forum (CRForum) on Wednesday, June 7, 2023 from 12:00 to 1:00 p.m. You must register to attend the Forum – please use the Zoom registration link here.
- Welcome and PPHS Reminders, Lori Jennex, MA, CIP, Senior Director, PPHS
- Mount Sinai Volunteers: Dana Kuefner, LMSW, CAVS, CVA; Director, Department of Volunteer Services Who they are, What they can do, How to get them on-boarded!
- Attaching and Updating Documents in RUTH– These are Must Haveskills!, Cyndi Roman, IRB Analyst II & Harrison Adler IRB Analyst I
- Questions/Concerns/Open Mic
Single IRB (sIRB) Requirements for Grant Submissions 5/15/2023
For all competitive federal grant submissions (i.e., new, competitive renewal, or resubmissions) that are multi-site or collaborative, the use of a single IRB (sIRB) may be required by Federal Regulation.
- If you would like ISMMS to consider serving as the sIRB for your project, P.I.s must consult with the PPHS regarding fees and include the cost in their budget.
- Please fill out the HRP-230 form and email to irb@mssm.edu for consideration at least two weeks prior to grant submission.
- If the ISMMS IRB agrees to serve as the sIRB, PPHS staff will provide sIRB fees to include in the budget and a letter of support from the PPHS Executive Director.
- Additional information is available here.
- If you would like to rely on an external IRB/institutional IRB other than ISMMS for a federally funded project, please consider the following:
- ISMMS IRB agrees to cooperate with the sIRB plan when the research is not a first in human trial and the sIRB is one of the following:
- Accredited and utilizing the SmartIRB Master Common Reciprocal Institutional Review Board Authorization Agreement (SmartIRB Agreement), or
- A non-accredited CTSA hub with no OHRP/FDA warning letters utilizing the SmartIRB Agreement
- An external IRB with which ISMMS already has an existing master agreement (WCG, BRANY, Advarra, Alpha, NCI, NMDP)
- Additional information is available here.
- ISMMS IRB agrees to cooperate with the sIRB plan when the research is not a first in human trial and the sIRB is one of the following:
Clinical Research Forum - May 3, 2023 - 5/1/23
Please join us, Wednesday, May 3, 2023, for the Clinical Research Forum (CRForum) from 12:00 to 1:00 p.m. You must register to attend the Forum – please use the Zoom registration link here.
AGENDA
•Welcome, Lori Jennex, MA, CIP, Senior Director, PPHS
•ClinicalTrials.gov – Conquerable or Madness? – Angela Lee, MPH, MS, PA, Clinical Trials Analyst: Samuel Pugo, BS, Project Manager: Litzzy Lugo, BS, Research Coordinator
•Meet the PPHS Team and Hear Their Best Tips for Processing Human Subjects Projects!
•Questions/Concerns/Open Mic
Clinical Research Forum - April 5, 2023 - Big News! 4/4/23
UPDATED Information:
Please join us, tomorrow, for the Clinical Research Forum (CRForum) on Wednesday, April 5, 2023 from 12:00 to 1:00 p.m. You must register to attend the Forum – please use the Zoom registration link here.•Welcome and PPHS Reminders, Lori Jennex, MA, CIP, Senior Director, PPHS
•eDMS Update, Haemar Kin, Senior Director, FCOI/Deans Office
•Big News! Cybersecurity Risk (formally InfoSec) Process Changes – Third-Party Risk Management (TPRM) Process Efficiencies, Thomas Wolff, IT Director, Software Security Assessments
•Questions/Concerns/Open Mic
Retirement of iOpen e-Consenting Application 3/30/2023
Dear Research Community,
Based on the Digital and Technology Partners (DTP) broadcast communication sent today at 9:00 a.m., if you are using iOpen with your IRB approved human subjects projects, please keep in mind that a modification via RUTH to your project is required to transition from iOpen to REDCap or other system you choose to use. For questions you may have on this transition, please email IRB@mssm.edu for assistance.
[PPHS/FCOI] Notice - FCOI System Update and the Effects on Your RUTH Submission 3/22/2023
To: Human Subjects Research Community
From: The Program for the Protection of Human Subjects (PPHS) and Financial Conflicts of Interest Committee (FCOIC)
As you have heard, on 4/10/23 the new eDisclosure Management System (eDMS) will replace the current Sinai Central system for managing financial disclosures and conflicts of interest. Please be sure to review the information and deadlines related to this change in Dr. Nestler’s email message dated 2/21/23.
Important RUTH Fact: If, you have started a project in RUTH (in the Pre-submission state), and the project is using an IF# from Sinai Central created between 12/01/2021 – 03/31/2023, you’ll need to either:
- submit the complete application (with all necessary forms) for IRB review by 4/4/23 at 4:30 p.m. or
- discard the submission and begin again starting in the eDMS on or after 4/10/23.
Only BRANY submissions where BRANY is negotiating the contract are not affected by the eDMS migration.
If you have questions about your project’s submission write to IRB@mssm.edu or for information about the new FCOI process write to Conflicts.of.Interest@mssm.edu.
Changes to the Smart Form in RUTH 3/17/2023
To support more robust reporting and assist users, the Program for the Protection of Human Subjects (PPHS), Tisch Cancer Institute (TCI), Research Administration, and Research IT have collaboratively made changes to the RUTH electronic smart form. With these changes, you will see new questions, a new date field for R2Rs (external IRB submissions), and updated help text. These changes will be in effect, Thursday, March 30, 2023. If you have any questions, please contact PPHS at IRB@mssm.edu or 212-824-8200.
Effective TODAY - New Informed Consent Template Required 2/15/2023
Effective TODAY – The IRB will only be approving consent forms using the new template (ver. 11/11/22). If your study is actively enrolling, you will be required to transition to the new consent template at the time of your next modification that impacts the consent form or at the time of your continuation submission, whichever comes first. For guidance on this process, please see the announcement below on 1/17/23 or contact the PPHS at IRB@mssm.edu
Change in Process for NIH Certificates of Confidentiality (COC) 2/15/2023
The NIH recently revamped its approach to the issuance of Certificates of Confidentiality (COC) for projects that are not receiving Federal support. Guidance around the issue is found here.
The characteristics of projects that they are willing to protect has become limited and is less than perfectly clear. For that reason we strongly urge you to work with the PPHS before submitting your application or responding to any inquiries from the NIH COC coordinator.
Please contact the PPHS at irb@mssm.edu and be sure to put COC in your subject line,
Thank you in advance for your anticipated attention to this matter.
Learn How to Transition to the New Consent Form Template with the PPHS. Register Now! 1/24/2023
Do you have studies that are actively enrolling participants? Yes? Then, this is for you! The PPHS is hosting a series of 2022 consent form transition info sessions. Attend one or more of these sessions and learn all that you need to know to ensure a smooth transition of your existing consent form to the new consent form. Come ready to ask lots of questions! The session dates are as follows:
Register for Friday Morning Sessions here!
Friday, 1/27 – 10AM – 10:30AMFriday, 2/03 – 10AM – 10:30AMFriday, 2/10 – 10AM – 10:30AMFriday, 2/17 – 10AM – 10:30AMFriday, 2/24 – 10AM – 10:30AMFriday, 3/03 – 10AM – 10:30AM
Register for Thursday Afternoon Sessions here!
Thursday, 2/09 – 2:30PM – 3:00PMThursday, 2/16 – 2:30PM – 3:00PMThursday, 2/23 – 2:30PM – 3:00PMThursday, 3/02 – 2:30PM – 3:00PM
Make sure to register early and often. For any questions about these sessions, contact Marilyn Eshikena (marilyn.eshikena@mssm.edu).
See you there!
New NIH Policy on Data Management and Sharing Plans - Does Your Project Meet the Requirements to Share Your Data? 1/24/2023
Effective Wednesday, January 25, 2023, the National Institutes of Health (NIH) requires a data management and sharing plan for competitive grant applications that are due on or after the effective date. Please follow the NIH and GCO guidance on creating a data sharing and management plan for your project at the time you submit your grant application. After your grant is awarded, NIH will require their certification forms to be completed indicating how your data will be uploaded, whether there are data use limitations, etc. The PPHS is the signatory official for all NIH human subjects data sharing certifications required for sharing data with NIH.
As you craft your data sharing and management plan for your grant application, keep in mind:
- What you submit to the PPHS, including the protocol, HRP 503, consent and data sharing plan all must be aligned.
- That what you submit to the NIH is consistent with Mount Sinai Institutional policies.
- Be sure that what you propose in your plan can be operationalized.
- Data use limitations, based on the type of data, may apply.
- That you cannot require participants to grant authorization for unrestricted future use of their data/samples if the project holds out the prospect of potential benefit.
- Review the 11/11/22 consent template for instructions on language to use when creating your project’s consent forms.
You can view PPHS guidance and access to NIH certification forms posted on our Guidance webpage here. Also, be sure to review and follow the PPHS Future Use Policy and the New York State Section 79-L Confidentiality of Records of Genetic Tests, Civil Rights (CVR)
Effective 2/14/2023 - New Requirements for Human Subjects Research - Updated Informed Consent Form Template 1/17/2023
Per the PPHS notification provided on 12/6/2022 (see the Research Road Map), there is a new Informed Consent Template (ver. 11/11/22) available for use.
As a reminder, as of 2/14/2023, the PPHS will not approve any consent forms on an outdated consent template for a project where Mount Sinai is the IRB of Record.
- Keep in mind that any submission that involves an informed consent form (ICF), currently in progress with the PPHS, must be approved prior to 2/14/2023 if you are still using an older consent template.
- If you have a submission currently in progress, please be prompt in your responses to IRB queries and communicate with your IRB analyst if you have questions about the status of your project.
- Any project still pending as of 2/14/2023 will be required to transfer to the new template prior to approval.
- Please complete and attach the following forms (available in the RUTH Library > General tab) to your modification submission at the time of your consent form transition, if applicable:
- R2S Studies: Transfer parent study ICF master template and Mount Sinai site specific ICF and obtain IRB approval prior to transferring any external sites’ consent forms to the new template.
The transfer date of 2/14/2023 is less than six weeks away. Based on the posted PPHS service pledge any submission not yet submitted to PPHS will likely not be approved prior to that deadline and therefore must be submitted using the new consent template. Please contact your IRB analyst or the general IRB inbox at IRB@mssm.edu if you have any questions about this requirement.
FOR STUDIES UNDER AN EXTERNAL IRB (R2R)
For new initial R2Rs, please begin using the revised HRP-232R (version 01.09.2023 available in the RUTH Library > General tab). Please note: negotiations are still in process with NCI CIRB. A separate notification/instructions will be sent when that process is complete.
- New initial R2R submissions submitted on/after 1/23/2023 will be returned if submitted using an older version of the HRP-232R unless an exception has been granted*.
- *If you are currently actively negotiating with the sponsor, external IRB or PPHS, please email Jennifer Kucera (Jennifer.kucera@mssm.edu) to discuss whether an exception can be granted for your study.
- Studies that PPHS has released to submit to an external IRB (in the Pending sIRB state in RUTH), will automatically be granted an exception.
- For studies currently in Pre-Review in RUTH, the PPHS will evaluate and communicate with the study team about the need to switch to the revised HRP-232R/2023 ICF language on a case-by-case basis.
- The revised HRP-232R (version 01.09.2023) will now be the IRB Waiver of Jurisdiction that gets signed by PPHS for WCG, Advarra, BRANY and Alpha IRB.
- The separate Waiver of Jurisdiction will no longer be required.
Existing R2Rs
For studies that are open to enrollment under an external IRB, certain language will need to be 1) updated on any applicable ICF(s), 2) approved by sponsor and the external IRB and 3) submitted to PPHS (at the time of another modification or Continuing Review, whichever comes first).
Please complete and attach the appropriate form (available in the RUTH Library > General tab) to your submission at the time of your consent form transition:
- HRP-235C: R2R Consent Transition for Existing Studies (General)
- HRP-235D: R2R Consent Transition for Existing BRANY Studies
- HRP-235E: R2R Consent Transition for Existing Studies under the NCI CIRB: Please note: negotiations are still in process with NCI CIRB. A separate notification/instructions will be sent when that process is complete.
Human Subjects Research Related to the NYSNA Strike 1/9/2023
With the start of today’s labor action impacting various hospitals within the Mount Sinai Health System, the PPHS would like to emphasize some guidelines to the research community.
- Research participant safety is paramount and takes precedence over other considerations. Protocol deviations of protocols to assure participant safety do not require prior approval by the IRB.
- Multiple research protocol deviations are expected during this period but wait to report them to the PPHS until we issue further guidance. Adverse events should be reported promptly per current policies.
- Please keep your sponsors aware of the situation.
- Please be mindful that the use and administration of investigational agents will require specific training and likely supervision of replacement personnel. The study sponsor and/or the holder of the IND/IDE will be an important resource.
- Site visits by sponsors, CRS’s, etc. should be rescheduled.
- For those studies using an external IRB please know that the PPHS has already notified the four largest external IRBS; BRANY, WCG, Advarra and the NCI IRB of the possible strike. That covers about 85% of external IRB projects. All PIs should keep the specific external IRB, responsible for their projects, updated and certainly notify them of any deviations etc., as required.
It was just about three years ago that research activities within the Mount Sinai Health System were severely impacted by the pandemic. We went through that unprecedented disruption safely and with the vast bulk of research activities successfully continued and/or resumed. We strongly hope this disruption will be much shorter and less disruptive. The PPHS will continue to do what we can to support our research participants, and our faculty and staff during this period. Please contact us by email (IRB@mssm.edu) or phone 212-824-8200 as needed.
RUTH Alert - New Submission Option in RUTH - Effective 11/11/22 at 9:30 am
Beginning 9:30 am, November 11, 2022, you will have the option to create and submit a combined Modification and Continuing review submission (MODCR combo) in RUTH. The steps to access this option are the same steps you currently follow to create a Continuing review submission or a Modification submission. Below are the steps to follow to submit a combination MODCR combo application:
1. Select Create Modification/CR from the parent study workspace.
2. On the screen that follows, you will have three options to choose from. To create a combined MODCR combo, select the third option: Modification and Continuing Review.
3. Under Modification scope, select either Study team member information or Other parts of the study, depending on the type of Modification you would like to combine with your Continuing review. You can also select both Study team member information and Other parts of the study.
If you do choose to submit a MODCR combo, please ensure that you submit these AT LEAST six weeks in advance of your project’s expiration date. If the modifications you are making are substantial and require multiple rounds of review, this may impact the timing of when the submission can be processed which could result in a study lapse if the PPHS is not given adequate time to review the application.
If your project is greater than minimal risk, your MODCR combo will always be sent to a Board meeting, regardless of how trivial the modifications you may be making. Therefore, in most cases, it is still recommended to submit modification and continuation submissions separately.
The MODCR combo submission is not an option for Request to Rely (R2R) studies.
R2S (studies where Sinai is the sIRB):
The MODCR combo will be an option for the Parent Study. In this submission, you will be able to submit the following:
– Continuing Review documents for all sites involved in the research under the oversight of the Mount Sinai IRB
– Modifications to the parent study (updating overall study, study ICF master template and/or study recruitment master templates
– Modifications to the Mount Sinai site(s) (updating Sinai personnel, locations, Sinai-specific ICFs, Sinai-specific recruitment materials)
The MODCR combo submission will not be an option for external sites.
– Any external site modifications will continue to be submitted using the Create Site Modification activity.
– The Continuing Review for external sites is completed at the same time as the Continuing Review for the parent study by attaching the HRP-233 for each external site.
If you are uncertain as to whether the new MODCR combo will work for your specific situation, consult the IRB analyst assigned to your parent project before beginning your submission.
Happy Birthday RUTH + Big News - 9/23/2022
Happy 2nd Birthday RUTH!
We are happy to celebrate September 23rd to mark our second year of using RUTH – our electronic application submission system. Thank you to everyone for your continued support.
Big NEWS:
1) The PPHS has completed its review of the informed consent template (RUTH form HRP-502a) that has been in use for several years. While we updated the consent form when the changes to the Federal Regulations occurred in 2018, we’ve fine-tuned the document to reflect other changes from state, federal and school policies and practices. Today we are sharing the updated version and seeking comments by the ISMMS research community. Please have your comments submitted to the PPHS by October 7, 2022. Click here to begin the review process.
2) The PPHS has updated the Recruitment Guidelines to include information on recruiting (peers, colleagues, and subordinates such as students or employees). The updated guidelines can be found on the PPHS website’s Guidance page here under the heading Screening and Recruitment.
Important Reminders:
1) The PPHS requires 6 weeks to process applications. Be sure to plan accordingly. There have been a high volume of last minute submissions which creates congestion in the flow of processing applications and affects everyone.
2) Industry sponsors must see and accept changes proposed by you/the research team prior to returning the application back to the PPHS.
3) For all Investigator Initiated Trials, please add IIT in the short title of those studies when there isn’t a budget for IRB fees. Currently the PPHS doesn’t invoice for most IIT but if we don’t see IIT in the title its likely an invoice will be sent out. If you are able to negotiate the PPHS review fees into the budget for your IIT, please do so. In the near future, PPHS will be reassessing our current practice.
4) Please don’t use macros in your Word documents – they cause RUTH to get upset and affect our ability to conduct a review.
5) Study Personnel Changes guidance last issued on February 10, 2016 was deactivated when we rolled out RUTH in September 2020. Since then, all personnel working on a project must be approved by the IRB prior to them starting any work. The Modification Submission checklist is posted in the RUTH library.
New PPHS RUTH Policy, New Check Lists and Reminders - 10/22/2021
New PPHS Policy on IRB Submission Withdrawals
To speed up operations and to support better reporting efforts, the PPHS is implementing a new policy related to stale application submissions. An application is considered stale if the research team has not acted on the PPHS requested changes in 30+ days (equal to receiving two Response Time Exceeded notices in RUTH).
Effective Monday, 11/1/2021, applications submitted* through RUTH will be withdrawn if they have been in a state of “clarifications requested” for 30+ days (i.e. the research team has received two Response Time Exceeded reminders from RUTH as listed on the History tab). To be clear, your submission will not be discarded, and the project may be resubmitted to RUTH once the requested changes have been made.
*RNI submissions are not included under this new policy.
IRB Submission Check Lists are HERE!
Application submission check lists are now available to assist you when making a request for IRB review in RUTH. These new check lists can be found in the RUTH Library under the Checklist tab.
PPHS Research MRI Policy
As a reminder, effective last year (12/21/20), PPHS updated the guidance on research MRIs. Please visit our PPHS Guidance page for the policy.
Please remember that for metallic objects screening, if imaging with ionizing radiation (i.e., CT or radiography) is needed to identify retained metallic foreign body(s), full Board review is required along with a submission to Radiation Safety. If ad hoc screening CT or radiography is needed, and this imaging was not disclosed in the initial protocol and consent, an amendment must be submitted to the IRB and Radiation Safety before the screening is performed. This pathway will rarely be approvable in children and in control groups.
Ancillary Review(s) Worksheet for Modifications in RUTH
Some modification submissions require certain ancillary office reviews. In order to assign the correct ancillary offices to review your modification submission, please use the HRP-388 worksheet as a guide. You are not required to upload the completed worksheet to your RUTH application. This worksheet is located in the RUTH library, on the Worksheets tab.
Happy Birthday RUTH + More - 9/29/2021
Happy Birthday RUTH!
We are happy to celebrate September 23rd as the one year mark of using RUTH – our electronic application submission system. Thank you to everyone for your continued support.
Updated Process when Submitting Investigator Brochures:
Any update to an Investigator Brochure (IB) (regardless of whether the change is administrative or introduces a new risk) must be submitted to the PPHS as a modification submission. These IB updates should no longer be submitted as a Reportable New Information (RNI) submission.
NIH Data Sharing Plans:
If you are submitting a Federal grant that requires a data sharing plan, we encourage you to check in with the PPHS before submitting your grant to be sure your plan is acceptable. If it isn’t, you will have stumbling blocks to maneuver, which will likely cause delays.
Request to Rely (R2R) – Using an External IRB:
It is PPHS policy to have all human research projects registered in RUTH regardless of which IRB has reviewed the study. All projects must use RUTH to register and must be updated with each of the study’s continuing review approvals. Without a continuing review registration, your project may be in Institutional non-compliance since FCOI, GCO and education requirements are reviewed institutionally, not by the external IRB.
Lapsed Studies or R2Rs Without Current Registration:
For all projects wishing to 1) continue work but have lapsed, 2) a continuing review has not been registered with the PPHS (R2R), or 3) your application was submitted too close to the expiration date, in addition to the continuing review, you will now also be required to submit a Reportable New Information (RNI) form explaining the reason for your delayed submission.
Reminders to Make Your Submission in RUTH Go Well
A Tidbit on How to Submit – After you click Finish in your RUTH application, you must click the submit button in RUTH for your submission or response to be sent to the PPHS for review. If you don’t hit SUBMIT, the PPHS won’t be able to act on your request. If the submission status is in a state of Pre-Submission, Clarifications Requested or Modifications Required, the project is not in the PPHS’ review queue. Remember 2 clicks does the trick – Click Finish AND Submit.
PI vs. Proxy: Only the PI or their proxies can click the submit button in RUTH. Teams should not be using the PI’s credentials to log in (a violation of IT policy) but rather ask the PI or the PI proxy to hit the SUBMIT button. If there are no proxies listed, contact the PI to assign one for the study.
Multi-Site or Single-Site Study: During the RUTH migration there was some confusion on how to answer the question “What kind of study is this?” Multi-site or Collaborative study OR Single-site study on the RUTH smart form. For clarity, use Multi-site/Collaborative when your study is one of many sites involved (more than one site outside of the Mount Sinai Health System). Use Single-site when Mount Sinai (including any of the system sites) is the only site(s) involved.
To Replace or Delete, That is the Question!: Remember when changing documents in RUTH, upload a revised, clean version of the document using the update button on the RUTH Smart Form (please do not remove or delete the older version of the document; use the update button to link the new, revised version with the older version).
Updates from the PPHS - 07/02/2021
Important News Updates from the PPHS
Starting Up Projects Previously Stopped due to COVID19:
As research continues to ramp up, the PPHS would like to remind you that if your human subjects research project was halted or modified due to the COVID19 pandemic, and you are ready to begin again, you should follow this guidance:
– If you submitted a modification to change or stop your project and that language is no longer accurate, you will need to submit a modification in RUTH.
– If you submitted a Reportable New Information (RNI) form, submit an RNI in RUTH to reverse your request.
-If you didn’t change any part of your study or report to the PPHS changes to your study, but rather voluntarily stopped the project, please inform the IRB via the narrative progress report memo included in your Continuing Review application.
Extended Expedited Expiration (EEE) of a Project – 3 Year approvals for qualifying projects:
Effective 4/14/21 the first phase of transitioning pre-2018 human subjects (HS) projects to the 2018 Common Rule has commenced. This means that eligible HS projects may now qualify for a 3 year approval at their next continuing review. This is such an exciting change for us. To be eligible for Phase 1, a study must meet all of the below criteria:
– Approved under Expedited review categories 2, 3, 4, 5, 6, and/or 7 as shown on the approval letter.
– Not banking specimens or data for future research or for sharing with other researchers for future use.
– The study team does not plan to close out the study in the following year.
– Study was granted a waiver of consent (i.e. is not consenting subjects) OR study is now closed to enrollment (i.e. no longer consenting subjects).
Each EEE application will be reviewed and acceptance is not guaranteed. Factors as to the sensitivity of the data, location of sites, etc. will all be considered in a final determination. If your project meets the qualifications and the reviewing analyst makes the determination the project qualifies, they will ask study teams to complete the HRP-420 checklist found in the RUTH Library and upload the completed checklist as part of their continuing review application. Speak with your assigned IRB analyst for further information.
Administrative – Non-FCOI Education Requirements – Reminder
While the PPHS requires CITI Program education modules for those involved with human research, the Administrative – Non-FCOI role in RUTH (which does not currently require the IRB education modules), should be used very infrequently and only for the following team members:
– Team members who are solely providing administrative support and
– Team members are not ever involved in interacting with potential or enrolled human subjects (including managing reimbursements, acting as the contact for the project for subjects, etc.) and
– Team members are not ever involved in collecting, analyzing or managing data.
Also, this role does not negate the FCOIC’s requirement for meeting their education requirements in the CITI Program.
Forms Reminder:
As a reminder, please download forms from RUTH rather than saving forms on your computer and reusing them. The PPHS would like you to be using the most updated forms in your applications.
Wishing you a safe 4th of July holiday!
Updates from the PPHS - 05/05/2021
IRB University
IRB University is now available through PEAK. Courses include the topics below. Please visit PEAK and search IRB to find these courses.
IRB 101 Basic Steps of the IRB Submission
IRB 201 Protocol and Consent
IRB 300 Series for Request to Rely (R2R) Submissions
IRB 400 Series for Request to Serve (R2S) Submissions
PPHS Office Hours are Expanding
PPHS office hours are expanding to include Mondays from 10:00 am to 11:00 am beginning May 10th. Use this link to join on Mondays. Use this link to join on Wednesdays between 2:00 pm and 3:00 pm.
Emergency Investigational New Drug (eIND)
As a reminder if you are a physician using an eIND, please visit the RUTH Library and under Worksheets, use the HRP-322 – Emergency Use for guidance and instructions. eIND uses must be reported in RUTH as Reportable New Information (RNI) within 5 business days of the use.
Faculty Corner:
As a reminder, research using human subjects will most likely require a submission in RUTH to PPHS. Even if you think your project qualifies as exempt, only the PPHS can make an exempt determination so be sure to submit in RUTH. If you’re using secondary QA/QI data, and are requesting IRB approval, be sure to include your QA Committee determination in your application.
RUTH Upgrade
RUTH is going through a minor upgrade. On May 10th, you’ll notice a change to your workspace in RUTH with a new tab called Dashboard. On that new tab you’ll be able to see projects you’ve recently viewed on the left side of your screen. There’s also a new way to begin an IACUC and IRB project in a handy space saver tile but you can also continue using the IRB tab.
RUTH will be unavailable from 7:00 p.m. Friday, 5/7/21 through 9:00 a.m. Monday, 5/10/21. Thank you for your patience during this time.
Information You Need From the PPHS! - 04/07/2021
- Expedited Extended Expirations (EEE) offer eligible minimal risk studies a three year approval. To learn more, log into the Clinical Research Forum, TODAY – Wednesday, 4/7/21 at Noon. Register here!
- RUTH has a new Ancillary Office for Health and Hospitals Corporation (Elmhurst and Queens sites). If you’ll be working at either site, please add them on the local sites tab and as an ancillary office for review.
- Mount Sinai will only rely on AAHRPP-accredited IRBs. During your NIH grant submission, please ensure that the IRB serving as the single IRB (sIRB) is AAHRPP-accredited. Please refer to ISMMS PPHS Statement on NIH sIRB Policy.
- Research collaborations with external sites (private practices, clinics, institutions) may require IRB approval for the external sites. If the site does not have an IRB, please submit the following for PPHS consideration to serve as the IRB of Record:
- Determine if the site is engaged in the research by using HRP-311 – WORKSHEET – Engagement Determination.doc(0.05)
- If the site is engaged, email irb@mssm.edu the following information:
- The completed HRP-311
- Summary of the research, including which research activities will be done at each organization.
- List the funding sponsor, if applicable
- Any agreements that exist between Mount Sinai and the external site, if applicable
- Please review the information on the PPHS R2S website prior to grant submission if:
- An sIRB is mandated for your NIH grant submission and
- You would like the Mount Sinai IRB to serve as the sIRB
- Reminder: PPHS has instructions for our Request to Rely (R2R) process listed here. Continuing review approval documents (letter, ICFs, etc) must be submitted in RUTH prior to the expiration date for local review. It is noted that continuing review approval from external IRBs are often received by the study team very close to the expiration date. As such, once the approval letter from the external IRB has been submitted to the PPHS, the submission will be pre-reviewed and a comment will be added to RUTH when permission has been granted for research activities to continue. Please refer to the Quick Guide for R2R Continuing Review in RUTH for the submission process.
Information from the PPHS - (03/16/2021)
Please see the following information from the PPHS office:
1) An updated MRI guidance document has been posted on the PPHS website effective December 21, 2020. Please review it here: https://icahn.mssm.edu/research/pphs/guidance.
2) Be aware there are new General Data Protection Regulation (GDPR) questions that have been added to the RUTH Ancillary Office form in Redcap.
3) The form Request for Access to PHI in Preparation of Research has been added to the RUTH Library under Checklists. This form should be submitted to the IRB@mssm.edu inbox by researchers who are requesting access to protected health information (PHI) in preparation for research projects.
4) Quick tips for managing ancillary reviews in RUTH:
· Do add FCOI ancillary review for continuations, initials and any modifications where personnel are changing
· For relevant ancillary reviews (e.g. FCOI, IDS, FACTS): Do select organization (not Person) and review type. Response required should always be “Yes”
· Do not update the ancillary review to show the review was accepted. (Please leave that to the ancillary office reviewer)
· Do not delete an ancillary review after an ancillary office submits their review
5) Lastly, PPHS has a 50-gallon fresh water aquarium, with fish, and cabinet previously owned by the late Dr. Jeffrey Silverstein. We would like to give this beautiful aquarium away to a Mount Sinai department who may find the same joy we have had watching these beautiful fish. The aquarium is currently being maintained by an ISMMS approved professional aquarium vendor. If you would like more information, please contact Lori.Jennex@mssm.edu.
PPHS Requires Standardized Naming Convention for Documents Uploaded in RUTH (01/26/2021)
Beginning February 1, 2021, the Program for the Protection of Human Subjects (PPHS) will require the use of a new document naming convention (see below) to be used with the RUTH application submission system. Changes to document names for migrated projects is not required unless a change to the document is submitted for review. The effective start date is 2/1/2021, however, study teams can begin immediately.
Format for the Structured File Name should be: File Type then Study Number then Study teams can add any wording (title, etc.) next, but should be somewhat descriptive including versions/dates, etc.
For dates use format: mm.dd.yyyy
Click here for the PDF version of the requirements.
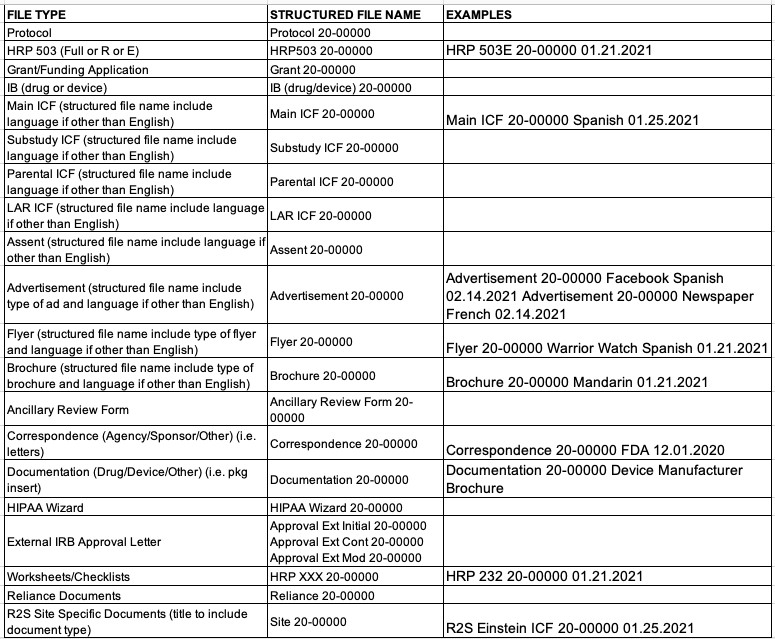
PPHS/IRB and RUTH Updates (12/11/2020)
The implementation of the new IRB application submission software system, RUTH, has been completed and was successful. We’d like to thank everyone for their support.
The PPHS would like to bring to your attention a few RUTH updates as well as updated PPHS processing and office updates.
- Proxy Update:
- A designated proxy can submit all projects (initial, continuing, and modifications) for a Principal Investigator (PI) once the proxy is granted the authority from the PI. Proxies are assigned per project and can only be added by the PI. When RUTH was implemented we originally instructed research teams that proxies could not submit initial studies but we can now confirm proxies can submit for any kind of project, once designated. As a reminder to all Principal Investigators, a signature by your proxy is considered to be the same as your personal signature. Oversight of your projects including submissions to the PPHS are ultimately your responsibility.
- Reminders:
- As the Ancillary Office (AO) Review form evolves there have been a few minor changes. Please be sure to use the most recent form in Redcap.
- If your project renewal date is in the near future, consider submitting your project before the holidays to get the process started.
- Processing Update:
- The Comparative Review process, for changing external funding sources, has been updated. There are now two ways for the review to occur.
- For requests to add new Federal funding to an approved project, a comparative review will be done by the PPHS.
- For requests to add new non-Federal external funding to an approved project, a comparative review will be done by PI attestation.
- The new form can be found in the RUTH Library under the General tab.
- The Comparative Review process, for changing external funding sources, has been updated. There are now two ways for the review to occur.
- PPHS Office News:
- Starting immediately, our main telephone line will be answered in real time (prior the calls were forwarded to voicemail). Feel free to call 212-824-8200 for assistance from the PPHS.
- We also offer office hours every Wednesday, from 2:00 p.m. to 3:00 p.m. (Join virtually at https://mssmhipaa.zoom.us/j/86232644872). Some sessions are altered in the month of December (12/16 has been changed to 12/17 and there is no session on 12/30). You can find office hours information on the Research Road Map at researchroadmap.mssm.edu/reference/walk-in-consultations/.
- The PPHS will be closed on Wednesday, 12/16/20 starting at 12:30 p.m. for our holiday staff retreat. Please plan accordingly.
PPHS RUTH - UPDATE (10/22/2020)
Trick or Treat? RUTH has been live for one month as of tomorrow, Friday 10/23/20, and to celebrate the roll-out success, we are offering only treats – no tricks this Halloween season.
- Friday, 10/23/20, at 5:00 p.m. will begin the last of the three migration waves to move project information and attachments from either Ideate or paper submissions into the new RUTH system. RUTH will not be available beginning Friday, 10/23/20 at 5:00 p.m. through approximately Monday, 10/26/2020 at 7:00 a.m. Please plan accordingly.
- On Monday, 10/26/20 you’ll be able to locate your project in RUTH by searching for the project’s HSM number. If you do not find your project, it’s likely it did not fulfill the migration criteria (contract not finalized, GCO submission not finalized, no response to PPHS revisions requested, project has an exempt or NHSR determination, the project approval expired) and you’ll need to start a new submission in RUTH. If you think your project should have been migrated and you can’t find it in RUTH, before you begin a new submission, write to IRB@mssm.edu and ask for assistance. Projects that will migrate are those that were or had a submission approved between 9/19/2020 through 10/23/2020.
- Below are three documents to assist you in using RUTH.
- A quick guide to adding Primary Contact, Proxies, and CVs > q guide ruth
- A guide for submitting a one time modification to update your migrated project information > mod guide ruth
- An attestation form to be used in the modification update for migrated projects > 2020 migration attest
- Reminders
- Remember to assign Ancillary Offices to your projects to start their review process as soon the project is submitted to the PPHS.
- Remember to hit the SUBMIT button (listed in the action links on the left side of the screen in RUTH)
- Check your CITIProgram.org accounts to be sure you have your Mount Sinai email and Life number listed in your profile and if you have multiple accounts to contact CITIProgram to merge them.
- Starting October 29, 2020, RUTH users who do not have their Mount Sinai email address in Sinai Central will not be able to access the system.
PPHS - RUTH is LIVE - UPDATE (10/02/2020)
Welcome to RUTH! We have heard a great deal of positive feedback about the new RUTH system and we couldn’t be happier. In fact, we want to hear all your comments, on all levels, so we’ve created a forum in which you can leave them. Visit RUTH Comments to leave your comments on the system. However this is not a site to request technical help so if you need tech support, please submit an OS ticket.
As with all projects where migration of data is involved, issues do pop up. Below you will find helpful information:
-
- PI Proxy Question: If your PI is interested in assigning a proxy, they need to identify someone already listed on the study team and open the approved study on the Active tab or on the In-Review tab (if it is still in pre-submission). On the left side of the screen, they will click add proxy, a window opens and they can choose one, many or all the people on the project to act in this role. Remember that the proxy role carries a great deal of responsibility as they can modify and submit on behalf of the PI. Only initial studies are required to be submitted by the PI.
- Upload CV: Everyone who will be working in the RUTH system is required to store their CV in their profile. You can do that by logging into ruth.mssm.edu, find your name on the right side of the screen and click it, then click My Profile, navigate to the left side of the screen to the Upload CV link and follow the prompts. No one else other than the user can upload their CV into the system.
- Ancillary Offices: As a reminder, study teams are responsible for assigning ancillary offices in RUTH so those offices can review the submissions, as needed. Use the Ancillary Form in RUTH to understand which ancillary offices are needed. FCOI must be assigned to all submissions with some exceptions (final reports, some BRANY studies, etc.) It is not necessary to assign ancillary offices, other than FCOI, for continuations.
- Missing Funding Sources: If you do not find your funding source in RUTH, you can write to irb@mssm.edu and ask them to add the name to the pick list.
- Migrated Projects: Remember for migrated projects, you’ll use the HSM# associated with your project to find it in RUTH. You can find the HSM# on your IRB approval letter. You can also search by PI first or last name. You will not find NHSR, Exempt, Final Reports, projects that are still being processed in Ideate/paper. Projects approved after 9/18/20 by the PPHS will migrate over after 10/23/20. Personnel for the majority of paper projects have now been migrated over into RUTH. If you need help finding your project, you can contact the IRB@mssm.edu box for assistance.
- Protocols/HRP 503 Applications: Remember there are three HRP-503 applications that you will choose from to use as you submit your project. See the RUTH Research Road Map page here and review the 8/10/20 and 9/22/20 memos to understand which documentation you need to use with your project. We’ve provided a link to an NIH Protocol wizard int he 8/10/20 memo for reference. This protocol template is not mandatory but provided as an example or could be used – each PI will need to decide what they will use but all protocols must have the required information.
- CITI Training: If your CITI training is not appearing for personnel on a project, plese make sure that all personnel have updated their institutional profile in CITI with their Mount Sinai email and life number.
Reminder – training sessions and drop in sessions have been scheduled for your convenience as we transition into this new system. Please take ample advantage of these sessions. You can find more information on when these sessions occur here.
PPHS - RUTH is LIVE - UPDATE (09/25/20)
On September 23, 2020 at 9:00 a.m., the new IRB application submission system went live. While the system was named RUTH in honor of Dr. Ruth Abramson the founder and esteemed leader of the PPHS, we note, with sorrow, the passing of another great Ruth, Justice Ruth Bader Ginsberg.
As with all go-live projects coupled with a migration component, a few items are being finalized along with a few reminders:
- Remember when looking up projects in RUTH, you’ll use the HSM# associated with your project.
- Remember that exempt determinations were not migrated over, so you won’t see them.
- Remember there are three HRP-503 applications that you will choose from to use as you submit your project. See the RUTH Research Road Map page here and review the 8/10/20 and 9/22/20 memos to understand which documentation you need to use with your project.
- You’ll see under My Profile –> Properties tab –> Courses Completed, a “date expired” of 12/31/1969 in some cases because some CITI courses do not have expiration dates and CITI automatically assigns that date as a place holder. You can disregard those dates.
- From the migration, all paper projects, where study team members were not listed electronically in the old system, did not get migrated into your RUTH project(s). Consequently, none of the study team members, except the PI, can view those projects. Research IT is working on a patch to migrate teams from paper projects into RUTH starting Monday, 9/28 through Friday, 10/2. If on Saturday, 10/3 you still do not see your RUTH project, and you have confirmed with the PI there are no study team members listed, the PI will need to submit a personnel modification to add study team members beginning 10/5. Do not submit any personnel changes for these migrated paper projects during the week of 9/26 through 10/4. If you, for some reason, you have an urgent need that cannot wait the week, please contact the PPHS at IRB@mssm.edu.
- A new addition to the submission process is our HIPAA wizard. As a reminder the wizard is not needed if you are obtaining consent and a HIPAA Authorization (i.e. using a consent form) but rather when a waiver or alteration of HIPAA is being requested (record reviews, etc.)
We’d like to thank everyone who has contributed to the success of the RUTH roll out. Without the support of Dr. Dennis Charney, Dr. Rosalind Wright, Dr. Eric Nestler and all ISMMS leadership, Finance, and Research IT, this huge project would not have been possible. We are excited to work with the entire ISMMS research community in RUTH.
PPHS - Required Documents for RUTH (09/22/2020)
Required Documents for RUTH
As previously discussed at the CRForum in August and again in our correspondence sent on 8/10 and in RUTH training, there is an expectation that new studies submitted in RUTH and previously approved studies being migrated over to RUTH will complete a newly designed HRP 503 IRB Application form, in addition to a standalone protocol (if applicable).
Templates are available in the library section of RUTH and on the PPHS website.
What documents are required in RUTH?
| Submission Type | Required Documents |
| Exempt determination request | 503-E |
| Minimal risk with no subject interaction or intervention | 503-R |
| Minimal risk with subject interaction or intervention | 503-Full Protocol |
| Greater than minimal risk | 503-Full Protocol |
| Request to rely on an external IRB | Protocol HRP-232 |
Frequently Asked Questions
1) What is an acceptable protocol format?
RESPONSE: The project protocol can be provided by the sponsor, prepared for a grant, or written specifically for this application. We strongly recommend using the NIH template found here if you are creating your own protocol for your submission. Teams cannot use the PDF of their IDEATE application or their previously approved HRP 503 template as their project’s protocol.
2) Are there any scenarios when I would not be required to fill out this new documentation for migrated projects?
RESPONSE: Projects that have completed all subject interaction/intervention (or are in long-term follow-up) and are open only for data analysis, do not need to fill out an updated HRP 503 PPHS/IRB Application form or submit a standalone protocol.
3) When must these documents be updated?
RESPONSE: All new projects in RUTH will require this documentation. For migrated projects, the application must be updated with the appropriate HRP-503 application and an acceptable protocol at the time of your first continuation. Teams can update their documents at the time of first modification if they choose to. PPHS reserves the right to ask for a new 503 or protocol at any time based on the review of the project and will expect to see new forms when a substantial modification is submitted.
RAIT - From IDEATE to RUTH: An Update on Support (09/22/2020)
Dear All,
Starting at 9am tomorrow, 9/23, RUTH can be accessed by going to ruth.mssm.edu and using your SSO.
In order to assist with the transition to RUTH, the Research Administration IT team has uploaded training material on our departmental webpage (https://era.mssm.edu).
To access the training material:
Copy and paste the following link into your preferred web browser: https://era.mssm.edu/supported-applications/ruth/training-videos/
Support for RUTH
Need assistance with RUTH? Please submit a ticket via (http://osticket.mssm.edu/support). You may also attend a Walk-In Session:
Morning sessions: Tuesdays & Thursdays from 10-11am
Afternoon sessions: Tuesday & Thursdays from 4-5pm
Thank you,
Research Administration IT Team
IRB Submission System Update GO LIVE - 9/23/20 (9/22/2020)
The RUTH Portal is live tomorrow Wednesday, 9/23!
This week’s noteworthy news:
ruth.mssm.edu will be live tomorrow morning at 9:00 a.m.
- Thank you to everyone who signed up for training. Additional training sessions have been scheduled. Visit the Research Road Map and search for RUTH for more information. You can also search the term List Serv and sign up for notifications for research related information.
- We are interested in your feedback, suggestions and thoughts on the new RUTH system. Let us hear what you have to say – please click here!
- Migration is a very involved process and many projects have been migrated into the RUTH system. The first time you log into RUTH be sure to check your studies as all studies may not have had their drug /device information brought over. Drug/Device information is contained in the PDF from IDEATE or in your protocol template/sponsor protocol (for non-IDEATE projects) that is present in your RUTH protocol currently. Also, when you’re ready to submit a study modification, you will need to complete a few areas of your protocol for questions that are required but were not asked in previous applications.
- Keep in mind that projects still being processed in Ideate or by paper must be finalized by 10/23/20. Projects approved between 9/21 and 10/23 will migrate into RUTH the last week of October. You will be able to access all documents in Ideate until then.
- Continuation reminders for paper and Ideate projects will continue to be sent out from those systems so its important to know which system your project is in and what your expiration date is but the project may have already migrated into RUTH.
- Starting 9/23/20 the Study Personnel Changes guidance last issued on February 10, 2016 has been deactivated. The new process of adding study personnel will be done in the RUTH submission system. In the RUTH system there is a personnel modification choice that allows you to only add personnel with no other changes.
R2R Training in RUTH (External IRB Submissions)
We are days away from rolling out our new electronic IRB submission system, RUTH, on 9/23/20. We’ve been sending out information regularly to help with the transition.
Training sessions have been scheduled for R2R submissions, where study teams who rely on or plan to rely on an external IRB. It is a prerequisite that you have attended one of the RUTH training sessions prior to attending this session.
Please choose a time to attend training.
*The session will last approximately 30 minutes, though we have up to one hour to address any questions.
Links for registration:
- Monday, September 21 from 1:00pm – 2:00pm*
- Tuesday, September 22 from 3:00pm – 4:00pm*
- Thursday, September 24 from 1:00pm – 2:00pm*
Sessions max out at 100 people. If your whole team can’t attend, please be sure to send at least one person to the training session.
PPHS to End SubmIssion Deadlines for IRB Applications Beginning 9/23/2020
With the implementation of RUTH, the electronic application submission system on 9/23/2020, the PPHS will be ending its deadline submission policy and will begin accepting applications on a rolling basis with a pledge to complete its review within a fixed period. Of course, to meet that pledge requires the cooperation of the research team and the ancillary offices. In general, studies will be reviewed in the order in which they are received. IRB submission review times vary, as they are based on the regulatory review categories, the complexity of the protocol and the completeness of the submission. Follow the below submission guidelines when planning your IRB applications:
Our Service Pledge
Full Board Review: Greater than minimal risk projects will be presented at a board meeting within 6 weeks of a completed application.
Expedited reviews: Minimal risk projects will be approved within 6 weeks of a completed application.
Please note that as we transition to the RUTH system, review times will vary for the first 4 to 6 weeks of this new process.
Implementation Guidance
New Projects and Modifications
- Can be submitted at any time
- Minimal Risk (MR) studies: Submit at least six weeks before your study’s proposed start date or date you wish to enact proposed changes.
- Greater than Minimal Risk (GTMR) studies: Submit at least six weeks before your desired IRB meeting date.
- TIP: For GTMR studies, use the attached 2020 IRB meeting schedule to determine the best meeting date for your project by counting back 6 weeks from the desired IRB meeting date.
Continuations
- Can be submitted at any time within the approval period. Please allow six weeks for review.
- TIP: Projects are approved for up to one year, so if you renew early, your subsequent approval will expire less than one year from the current expiration date.
- Don’t wait until the last minute to submit your continuation and risk your study expiring!
- Minimal risk (MR) studies: submit at least 6 weeks prior to your study’s expiration date.
- Greater than Minimal Risk (GTMR) studies: Submit at least 6 weeks before your desired IRB meeting date.
- TIP: If a study team wishes to keep the same expiration date every year, this must be noted in the application. This would primarily be limited to NIH grants. The PPHS is able to link or de-link the approval periods, if requested.
2020 IRB Meeting Dates
| Board | IRB Meeting Date |
| C | 9/22/2020 |
| D | 9/29/2020 |
| A | 10/6/2020 |
| B | 10/13/2020 |
| C | 10/20/2020 |
| D | 10/27/2020 |
| A | 11/3/2020 |
| B | 11/10/2020 |
| C | 11/17/2020 |
| D | 11/24/2020 |
| A | 12/1/2020 |
| NO MEETING** | |
| C | 12/15/2020 |
| D | 12/22/2020 |
| NO MEETING** | |
| B | 1/5/2021 |
| C | 1/12/2021 |
| D | 1/19/2021 |
| A | 1/26/2021 |
| B | 2/2/2021 |
PPHS - IRB Submission System Update (09/11/2020)
Fall is right around the corner and so is the roll out of the new IRB application submission system, RUTH! Wednesday, September 23rd is the go live date!
As of Friday, September 4th at 5:00 p.m., PPHS no longer accepts new submissions (initials, continuations, or modifications) through Ideate or via paper. For those who submitted their initial projects or responses required for approval before that deadline, the PPHS is working as fast as possible to move the reviews along – we ask that you also do the same when you receive an inquiry about your project. Remember all other office reviews, such as FACTS, MSIP, GCO, FCOI, InfoSec, etc. will need to be completed before the PPHS can release your project approval, so don’t forget to finalize those processes too.
All projects in Ideate or paper, submitted before that deadline, will need to be finalized between 9/5 and 10/23 in order to be migrated to RUTH during one of the scheduled migration dates. As a reminder, for all projects not approved or a determination not made by 10/23, the project will forever be left incomplete and to resurrect them, a completely new submission in RUTH will be required.
This week’s noteworthy news:
- All required reportable new information submissions (HRP 224 reports) will need to be submitted to the IRB Inbox (irb@mssm.edu) via the paper form (found on the PPHS website).
- Consider scheduling some time with your research team, on or close to the go live date of Wednesday, September 23rd, to update your profile and assign proxies to your studies. All tasks are easy enough and required but because you’ll need to assign proxies per project, you may need a bit of focused time to do so. Only the PI can assign a proxy to a project. The proxy will have authority to make changes and submit projects on behalf of the PI once assigned (and after the initial submission has been made).
Researchers, coordinators and staff training began 8/17/20 for one hour webinar sessions via Zoom. Registration is open and training sessions are scheduled through Tuesday, 9/22/20 – sign up early and often! Links for registration: Morning Sessions Afternoon Sessions. If your entire team can’t attend, please be sure to send at least one person to the training session.
PPHS - Join Us Online! IRB University Classes - A New Day with RUTH - (9/16/2020 & 9/17/2020)
Did you hear? RUTH rolls out soon! Starting on Wednesday, September 23, all IRB submissions will be created and managed in RUTH. Our new IRB University Classes will show you how to become familiar with the submission and review requirements.
Register in advance for these webinars. You will receive a confirmation email after you sign up.
NEXT UP:
IRB 101 – September 16, 2020, 2:00 – 3:00 PM
Register Here: https://mssm.zoom.us/webinar/register/WN_ohQWl4nRQPiqL1uAi_D4zg
IRB 201 – September 17, 2020, 2:00 – 3:30 PM
Register Here: https://mssm.zoom.us/webinar/register/WN_VNHFZk9GS1C3iH8rxax9Og
What is RUTH?
As we move into the summer months, we are pleased to announce that RUTH, the new IRB electronic submission and application tracking and review software, is fully under development and is on track for her launch date of September 23.
 The new system is named after Dr. Ruth Abramson who is warmly remembered by all those who knew her at Mount Sinai Medical Center and School of Medicine for her lifelong commitment to research and teaching. It is fitting to name our new system, which will support the review of all human subjects research, after Dr. Abramson who was so deeply aware of the importance of human research protections.
The new system is named after Dr. Ruth Abramson who is warmly remembered by all those who knew her at Mount Sinai Medical Center and School of Medicine for her lifelong commitment to research and teaching. It is fitting to name our new system, which will support the review of all human subjects research, after Dr. Abramson who was so deeply aware of the importance of human research protections.
As a reminder to those who have offered their help and support as Champions, we will be in contact with you soon to begin the process of testing RUTH.
Despite the disruption due to COVID19, please know that with our continuing efforts over the coming months, and the ongoing support of the Mount Sinai research community, RUTH will go live on September 23. With that in mind, we remind you to submit your annual renewal applications by their assigned submission deadlines, and please close any projects that are no longer active. This will ensure timely review of your protocols and lead to a smoother transition to RUTH’s software system.
We will continue to keep you updated on everything RUTH is up to over the next few months.
Rosalind J. Wright, MD MPH
Dean for Translational Biomedical Sciences Icahn School of Medicine at Mount Sinai
Glenn Martin, MD, CIP
Senior Associate Dean for Human Subjects Research Icahn School of Medicine at Mount Sinai
Eric Nestler, MD, PhD
Dean for Academic and Scientific Affairs Icahn School of Medicine at Mount Sinai
RUTH Training Information
Summer is officially here and we are using it to mark the kick off of our teaching plan for RUTH. As you know RUTH, the new IRB application submission software system, is rolling out on September 23, 2020 which will be here before we know it! With that, we are moving into teaching mode and want to let you know there are a number of ways to get to know RUTH.
Below is information for you to sign up for classes in August to learn how to use RUTH. In collaboration with Research IT, the PPHS will host training webinars beginning on August 17th. Randomly selected webinars will have a drawing for the attendees and someone will win a small gift.
- RUTH Champions – To ensure a successful roll-out, we have recruited colleagues to donate 2 hours of their time in mid-August to learn the new system and then take their knowledge back to their departments and be available to help their colleagues, researchers, etc. If you are part of our Champion team, you’ll receive an email over the next few days inviting you to schedule your volunteer time via Zoom. Details will be in the email message. If you think you might want to be a Champion, please contact Lori Jennex (lori.jennex@mssm.edu) for more information.
- Researchers, coordinators and staff training will begin August 17th for one hour sessions via Zoom. Registration for classes is now open with additional classes being added over the next few weeks – sign up early and as often as you like!
Links for registration:
Morning Sessions
Afternoon Sessions - Drop-in Zoom sessions will begin after September 23rd for direct hands on help with RUTH, the PPHS and Research IT staff.
PPHS - RUTH - IRB Submission System Update (8/31/2020)
Did you hear? RUTH, rolls out on Wednesday, 9/23/20. RUTH will sit in the cloud, fully secure, and without the need of having to use VPN/Tunnel to gain access. Hooray!
This week’s noteworthy news:
- Earlier this month we mentioned CITIProgram.org (where your education requirements for human subjects research are stored) will begin to download your information directly to RUTH starting Wednesday, September 23rd. In order for your information to load correctly in RUTH, you need to be sure your Life Number and Mount Sinai email address are listed. To double-check your information is correct, please follow these instructions:
Go to https://www.citiprogram.org/
At the top of the page, click Log in Through My Institution
Scroll down to and click: Icahn School of Medicine at Mount Sinai which will take you to the SSO page for Mount Sinai, then log in
Under Institutional Courses/Icahn School of Medicine at Mount Sinai, click View Courses
Scroll to the bottom and click Update Institution Profile
Fill in your Institutional email address (mssm.edu, mountsinai.org, nyee.edu, nychhc.org, icahn.mssm.edu, chpnet.org) and life number under Employee Number.
- While you’re updating your information, you might want to also look at Sinai Central and Sinai 1 to be sure your information is listed correctly there too.
- You may have noticed the new question in Sinai Central when completing your IF packet about team members involved in the consent process. Be sure to answer that question for each person you add to the packet.
- Remember – All project submissions (paper and Ideate) will stop for a two week period between September 5th – September 22nd. No submissions will be accepted after the Friday, 9/4 at 5:00 p.m. deadline. Submissions accepted for review will continue through the review process and must be completed by the updated date of 9/18/20 to be part of the first wave of migrated approved projects into RUTH. September 23 – October 23 is designated as a one month extension to clear up any projects not yet managed.
- NOTE: For all projects not approved, a determination not made or not closed by 10/23/20 (paper or Ideate), they will forever be left incomplete and to resurrect those projects, a new submission in RUTH will be required.
Researchers, coordinators and staff training began 8/17/20 for one hour webinar sessions via Zoom. Registration is open and additional sessions have been added through Tuesday, 9/22/20 – sign up early and often! Links for registration: Morning Sessions Afternoon Sessions. If your entire team can’t attend, please be sure to send at least one person to the training session.
Dean's Office - Research workflow improvements on 9/23/20 (8/28/2020)
This is a reminder about research administration workflow changes that will be rolled out on September 23 along with RUTH (our new IRB platform) going live.
- School- (seed-) supported projects involving human or animal research will no longer go through InfoED and GCO. They will be processed directly through RUTH and eIACUC. This change will increase efficiency by reducing the number of steps and avoiding redundant entries. Department chairs and administrators will automatically be informed of such protocols and will alert the IRB or IACUC if they do not approve a particular protocol. (Note: Projects that do not involve animals or humans, or projects that require an endorsement by GCO – e.g., Data Transfer Use Agreement, study drug donation, etc., must continue to be submitted to GCO via InfoED.)
- There will be a new IRB protocol creation process in RUTH – the ability to auto-populate personnel and other data from Sinai Central IFs (Investigator Forms) with a single click. This will eliminate the current requirement of manual data entry into multiple systems.
- Ancillary Office (e.g. Radiation Safety, Biosafety, Investigation Drug Services, etc.) reviews will be independent of each other resulting in time efficiency during the protocol review process.
- A new “Protocol Wizard” will provide information on appropriate routing to these ancillary offices and guide study teams on required steps.
- A new FCOI Dashboard will provide financial conflict data to the FCOI Office in a transparent and user-friendly way.
- New technical interfaces between InfoED, RUTH, eIACUC, and Sinai Central will enable better workflows and significant improvements in administration, operations, and compliance in research for all future projects.
- DURC (Dual Use Research of Concern) questions will be in Sinai Central along with the IFs. (DURC involves studies of numerous infectious agents.)
All faculty, researchers, coordinators, and staff can register for training sessions via Zoom. You can register for classes here: Morning Sessions Afternoon Sessions.
Thank you,
Eric Nestler
PPHS - RUTH IRB Submission System Update (8/20/2020)
Surfs up, summer is here and RUTH, rolls out on 9/23/20. We hope summer has wrapped its arms around you like a warm, cozy blanket, your days are long and lovely and your masks are on. We’ve been sending out information regularly to help with the transition to RUTH.
This week’s noteworthy news:
- For projects that should be submitted during the 2 week system shut down (9/7 – 9/22) or any projects expiring through 10/31/20, you’ll want to submit them to the PPHS for IRB review now and they will not be rejected for being submitted too early. Remember that you are still required to have all education requirements met, FCOI completed and an Infoed submission started. Keep in mind the change of not having to submit School sponsored projects to Infoed doesn’t begin until 9/23.
- In RUTH, you will need to update your profile with your current CV or resume the first time you login so be sure your CV or resume is current.
- If your project is under review now or will be on 9/4, remember some of the conditional offices use a longer period of time to review projects (i.e. InfoSec, FCOI, etc.). If your project involves a conditional office that requires extra time for review its best to submit your project ASAP and get that process started now. Don’t jeopardize the migration of your project into RUTH by not having it approved by 9/18. If you think the project may be held up, consider waiting until the roll out to submit initial projects.
- Remember – All project submissions (paper and Ideate) will stop for a two week period between September 7th – September 22nd. No submissions will be accepted after the Friday, 9/4 at 5:00 p.m. deadline. Submissions accepted for review will continue through the review process and must be completed by the updated date of 9/18/20 to be part of the first wave of migrated approved projects into RUTH. September 23 – October 23 is designated as a one month extension to clear up any projects not yet managed.
- NOTE: For all projects not approved, a determination not made or not closed by 10/23/20 (paper or Ideate), they will forever be left incomplete and to resurrect those projects, a new submission in RUTH will be required.
Researchers, coordinators and staff training began 8/17 for one hour sessions via Zoom. Registration is open now – sign up early and often! Links for registration: Morning Sessions Afternoon Sessions. If your entire team can’t attend, please be sure to send at least one person to the training session.
Remember you can always find all the notifications about RUTH on the Office of Research Services Research Road Map at New IRB Platform – RUTH.
PPHS - RUTH IRB Submission System Update (8/10/20)
We are approximately a month away from the 9/4/20 deadline to submit an application in Ideate before that system stops accepting applications and about six weeks away from rolling out our new electronic IRB submission system, RUTH, rolls out on 9/23/20. We’ve been sending out information regularly to help with the transition.
Noteworthy News:
- If you joined the CR Forum last Wednesday you heard that applications within RUTH will require a protocol and a newly designed HRP 503 PPHS/IRB Application. This will be true for all projects needing IRB review, unless it is strictly a chart or specimen project with no contacts with research subjects. In that case, we will provide a combined research summary/application form. For the rest of the studies, submissions will require a written protocol which can be provided by the sponsor, or prepared for a grant, or written specifically for this application. Protocols should follow the generally accepted NIH format which can be found as well as a wizard here. We urge teams to familiarize themselves with this new requirement before new applications are accepted beginning 9/23.
- Human subjects education requirements won’t change with the RUTH rollout but the way the information feeds into RUTH is different. In order for the information to display correctly in RUTH, each person listed on a project must have their Mount Sinai email address and Life # listed in their CITIProgram profile. Visit CITIProgram.org, click Log in Through My Institution, at the top of the page, navigate to your member profile, and check the information listed.
- As we transition to RUTH the PPHS will only use one IRB number along with the IF and GCO numbers. Your migrated project will keep it’s assigned HSM number as the project’s identifier. Be sure to have that number available so you can easily search for your project in RUTH and cross reference projects if you currently use the Ideate number now. The HSM# can be found on your approval/determination letter sent by the PPHS team.
- Remember – All project submissions (paper and Ideate) will stop for a two week period between September 7th – September 22nd. That means no submissions will be accepted after the Friday, September 4th at 5:00 p.m. deadline. Submissions accepted for review will continue through the review process and must be completed by 9/22/20 to be migrated into RUTH. September 23 – October 23 is designated as a one month extension to clear up any projects not yet managed.
- NOTE: For all projects not approved, a determination not made or not closed by 10/23/20 (paper or Ideate), they will forever be left incomplete and to resurrect those projects, a new submission in RUTH will be required.
Researchers, coordinators and staff training begins August 17th for one hour sessions via Zoom. Registration is open now – sign up early and often! Links for registration: Morning Sessions Afternoon Sessions. Sessions max out at 100 people. If your whole team can’t attend, please be sure to send at least one person to the training session.
PPHS - RUTH IRB Submission System Update (7/24/20)
The electronic IRB submission system, RUTH, rolls out on September 23, 2020. While it may sound far into the future, its only about nine weeks away! We have some important information for you to read to prepare for the GO LIVE date:
- Project submissions will HALT for a two-week period in September to allow the PPHS time to process all projects under review and move them to a finished status. Access to Ideate to submit a review request will not be available. Applications/projects previously submitted and currently under review at that time and applicatons accepted for the 9/4 deadline will continue through Ideate. If you miss the 9/4 deadline or your application is incomplete and rejected (education requirements missing, no InfoEd submission started, missing paperwork, incorrect or missing IF#), you will have to wait for 9/23 to submit your request through RUTH.
- Important dates:
- September 4, 2020 at 5:00 p.m. is the last submission deadline for Ideate applications to be accepted
- September 7th through September 22nd no submissions will be accepted (including initial, continuation and modification applications, 224s, commercial IRB requests, etc.). Information on submitting time sensitive submissions (i.e. risk-related reportable new information) during the two-week shut down will be shared at a later date.
- September 23rd RUTH comes alive!
- Check your approval letter to know and plan for your submission window. Projects with a submission window of 9/7-9/22 can be submitted for the 9/4 deadline or will be prioritized in RUTH once submitted in the week of 9/23-9/25
- Its recommended that you assess your portfolio and close any projects that are finished
- IRB Meetings will continue as usual
- For projects submitted by the 9/4 deadline that do not get finalized before RUTH goes live, a 30 day extension through October 23rd will occur
- For all projects not approved, a determination not made or not closed by 10/23 in Ideate, they will forever be left incomplete in Ideate. To resurrect those projects, a new submission in RUTH will be required.
- Important dates:
- Researchers, coordinators and staff training begins August 17th for one hour sessions via Zoom. Registration is open – sign up early and as often as you like! Links for registration: Morning Sessions Afternoon Sessions
- Drop-in Zoom sessions for RUTH will begin after September 23rd for direct hands on help from the PPHS and Research IT staff.
Dean's Office - Research Workflow Improvements (7/23/2020)
Everyone,
We are delighted to share upcoming research workflow changes that should significantly improve our efficiency and make your lives just a little bit easier. I want to thank Ashish Narayan and Joseph Finkelstein, along with numerous leaders and administrative offices on campus, for their hard work over the past several months to enable the following changes, which will take effect on September 23, when the new IRB platform, RUTH, goes live.
- School- (seed-) supported projects involving human or animal research will no longer go through InfoED and GCO. They will be processed directly through RUTH and eIACUC. This change will increase efficiency by reducing the number of steps and avoiding redundant entries. (Projects that do not involve animals or humans, or projects that require an endorsement by GCO – e.g., Data Transfer Use Agreement, study drug donation, etc., must continue to be submitted to GCO via InfoED.)
- There will be a new IRB protocol creation process in RUTH – the ability to auto-populate personnel and other data from Sinai Central IFs (Investigator Forms) with a single click. This will eliminate the current requirement of manual data entry into multiple systems.
- Ancillary Office (e.g. Radiation Safety, Biosafety, Investigation Drug Services, etc.) reviews will be independent of each other resulting in time efficiency during the protocol review process.
- A new “Protocol Wizard” will provide information on appropriate routing to these ancillary offices and guide study teams on required steps.
- A new FCOI Dashboard will provide financial conflict data to the FCOI Office in a transparent and user-friendly way.
- New technical interfaces between InfoED, RUTH, eIACUC, and Sinai Central will enable better workflows and significant improvements in administration, operations, and compliance in research for all future projects.
- DURC (Dual Use Research of Concern) questions will be in Sinai Central along with the IFs. (DURC involves studies of numerous infectious agents.)
We will be incorporating these changes into the RUTH training classes. All faculty, researchers, coordinators and staff training will begin August 17 for one hour sessions via Zoom. You can register for classes here: Morning Sessions Afternoon Sessions.
Stay tuned for more information regarding these changes.
Eric Nestler
RUTH - IRB Submission System
Research 411 Portal
Still Need Help?
